KEY POINTS
- Given the importance of sleep for optimal health and performance, a number of nutritional interventions has been investigated to determine their effectiveness in enhancing sleep quality and quantity.
- As some nutritional interventions may exert effects on neurotransmitters that are involved in the sleep-wake cycle, it is possible that these interventions may enhance sleep.
- High glycemic index foods may be beneficial for improving sleep if consumed more than 1 h prior to bedtime and solid meals may be better than liquid meals at enhancing sleep.
- From the current literature, it appears that diets high in carbohydrate may result in shorter sleep latencies, while diets high in protein may result in improved sleep quality and diets high in fat may negatively influence total sleep time.
- Tryptophan, melatonin and valerian are other substances that have some scientific evidence for enhancing sleep.
BACKGROUND
While the exact function of sleep is not fully understood, sleep has extremely important biological functions. This is demonstrated by the negative effects that sleep deprivation can have on performance, learning, memory, cognition, pain perception, immunity, inflammation, glucose metabolism and neuroendocrine function. A number of nutritional substances have traditionally been associated with promoting sleep. Researchers have recently begun to investigate their effectiveness as a substitute for pharmacological interventions.
SLEEP OVERVIEW
Sleep Stages
Sleep can be defined as a reversible behavioural state where an individual is perceptually disengaged from and unresponsive to the environment (Carskadon & Dement, 2011). Sleep is a complex physiological and behavioural state that has two basic states based on physiological parameters. These are rapid eye movement (REM) and non-REM (NREM). An electroencephalogram (EEG), in which electrodes measures brain electrical activity, is used to identify the two states (Figure 1). NREM sleep is divided into four stages (1-4) which are associated with a progressive increase in the depth of sleep (Carskadon & Dement, 2011). REM sleep is characterised by muscle atonia, bursts of rapid eye movement and dreaming. Therefore, REM sleep is an activated brain in a paralysed body.
Measuring Sleep
There are two commonly used methods to assess sleep. The first is actigraphy and it involves monitors on the wrist, which are worn like a wristwatch that continuously record body movement (usually stored in 1-min periods), and the recording of sleep diaries, where participants record the start and end times and dates for all sleep periods (i.e., nighttime sleeps and daytime naps). Data from sleep diaries and activity monitors are used to determine when participants are awake and when they are asleep. Essentially, all time is scored as awake unless (i) the sleep diary indicates that the participant was lying down attempting to sleep and (ii) the activity counts from the monitor are sufficiently low to indicate that the participant was immobile. When these two conditions are satisfied simultaneously, time is scored as sleep. Actigraphy is useful for understanding sleep patterns as it is noninvasive and relatively easy to collect data over significant periods of time (commonly 2 wk of monitoring).
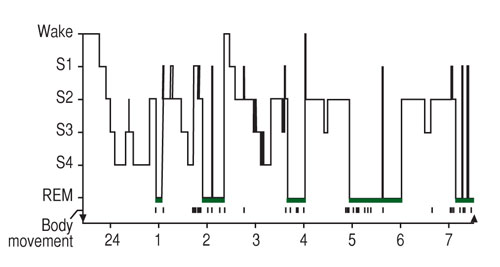
Figure 1. The progression of sleep stages across a single night in a normal young adult volunteer is illustrated in this sleep histogram. The text describes the ideal or average pattern (Carskadon & Dement, 2011).
The second method is polysomnography (PSG), by which body functions such as brain activity (EEG), eye movements (EOG), muscle activity (EMG) and cardiac activity (ECG) are measured. PSG provides information on sleep staging and is considered the “gold standard” for assessing sleep quality and quantity. PSG can be expensive, is labour intensive and is often used primarily for assessing clinical sleep disorders.
NUTRITIONAL INTERVENTIONS TO ENHANCE SLEEP
There are a number of neurotransmitters in the brain that are involved in the sleep-wake cycle. These include serotonin, gamma- aminobutyric acid (GABA), orexin, melanin-concentrating hormone, cholinergic, galanin, noradrenaline and histamine (Saper et al.,2005). Therefore, it is possible that nutritional interventions that act upon these neurotransmitters in the brain may also influence sleep.
Dietary precursors can influence the rate of synthesis and function of a small number of neurotransmitters, including serotonin (Silber & Schmitt, 2010). Figure 2 below depicts the means by which diet may influence the central nervous system and through the production of serotonin (5-HT) and melatonin. Synthesis of 5-HT is dependent on the availability of its precursor in the brain, the amino acid L-tryptophan (Trp). Trp is transported across the blood- brain barrier by a system that shares other transporters including a number of large neutral amino acids (LNAA). Thus, the ratio of Trp/ LNAA in the blood is crucial to the transport of Trp into the brain and an increase in this ratio can be achieved by the intake of pure tryptophan or tryptophan-rich protein (Silber & Schmitt, 2010). The food protein with the highest Trp content and most favourable Trp:LNAA ratio is a-lactalbumin, a whey-derived protein (Heine, 1999). Ingestion of other forms of protein generally decrease the uptake of Trp into the brain, as Trp is the least abundant amino acid and, therefore, other LNAA are preferentially transported into the brain. Carbohydrate, however, increases brain Trp via insulin stimulation of LNAA into skeletal muscle, which results in an increase in free-Trp (Fernstrom & Wurtman, 1971).
Carbohydrate
A small number of studies have investigated the effects of carbohydrate (CHO) ingestion on indices of sleep quality and quantity. Porter and Horne (1981) provided six male subjects with either a high CHO meal (130 g), a low CHO meal (47 g) or a meal containing no CHO, 45 min prior to bedtime. The high CHO meal resulted in increased REM sleep, decreased light sleep and wakefulness (Porter & Horne, 1981). However, the caloric content of the meals was not matched in this study making it impossible to tell whether the effect was due to the carbohydrate or the calories.
The effect of meal vs. drink (with high, normal and low CHO contents) vs. water at various time intervals prior to sleep has also been studied (Orr et al, 1997). Results demonstrated that solid meals enhanced sleep onset latency (time taken to fall asleep) up to 3 h after ingestion and the liquid meal was slightly better than water. There was no effect of meal or drink composition on sleep.
Afaghi et al. (2007, 2008) conducted two studies investigating CHO ingestion prior to sleep in healthy males. In the first study, high or low Glycemic Index (GI) meals were given 4 h or 1 h prior to sleep (Afaghi et al., 2007). The high GI meal significantly improved sleep onset latency over that of the low GI meal. In addition, providing the meal at 4 h prior to sleep was better than a meal at 1 h prior to sleep. In the second study, a very low CHO meal (1% CHO, 61% fat, 38% protein) was compared to a control meal (72% CHO, 12.5% fat, 15.5% protein) matched for energy, 4 h prior to sleep (Afaghi et al., 2008). The very low CHO meal increased the percentage of time spent in slow wave sleep (stages 3 and 4 of NREM), and the time spent in REM sleep when compared to the control condition. Finally, Jalilolghadr et al. (2011) provided eight children with either a high GI (200 mL milk and glucose) or lower GI drink (200 mL milk and honey), 1 h prior to bedtime. In this study, the high GI drink increased arousal to a greater extent than the low GI drink, suggesting a poorer quality of sleep.
From the limited and somewhat contradictory nature of the above studies, it appears that high GI foods may be beneficial if consumed more than 1 h prior to bedtime, and that solid meals may be better than liquid meals at enhancing sleep.
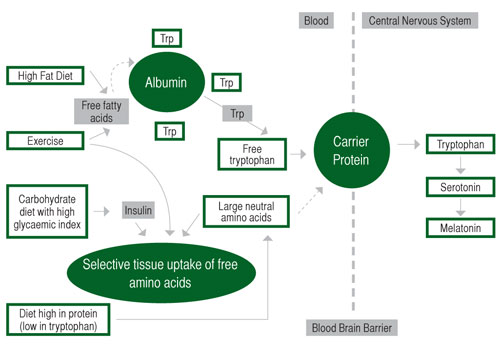
Figure 2. Effects of diet on tryptophan (Trp) uptake and the central nervous system. Adapted from Grimmett & Sillence (2005).
Acute Mixed Composition Meals
Only a small number of studies have investigated the effects of meals or drinks of varying composition on sleep. Hartmann et al. (1979) provided a drink with the evening meal which was either high fat (90 g), high CHO (223 g) or high protein (30 g). The findings revealed no effect of any of the drinks on sleep when compared to no drink. Zammit et al. (1995) examined the effects of high vs. low energy liquid meals (993.5 vs. 306 Kcal) provided at lunch, compared to no meal on daytime naps. Both liquid meals demonstrated increased time in stages 2 and 3 of NREM sleep when compared to no meal. However, there were no differences in sleep onset latency (Zammit et al., 1995).
Again, there is very limited research in this area, but it appears that reduced caloric intake may result in poor sleep.
Habitual Diet
The above-mentioned studies have examined acute nutritional manipulations on sleep. There has also been research conducted that investigated chronic manipulations or habitual dietary intake. Kwan et al. (1986) provided six healthy females with a low CHO (50 g/day) diet for 7 d and reported increased REM latency when compared to sleep prior to the 7 d intervention when the subjects consumed their usual diet. Lacey et al. (1978) also studied females for 7 d with either high protein (>100 g), low protein (<15 g) or normal daily protein intakes. Results showed that high protein intakes resulted in increased restlessness, while low protein intakes resulted in reduced amounts of slow wave sleep. However, there were no differences in total sleep time (Lacey et al., 1978). While it is difficult to draw definitive conclusions from this study, it is clear that altering daily protein intake may affect sleep quality.
In a recent comprehensive study, Lindseth et al. (2011) manipulated the diet of 44 adults for 4 d. Diets were either high protein (56% protein, 22% CHO, 22% fat), high CHO (22% protein, 56% CHO, 22% fat) or high fat (22% protein, 22% CHO, 56% fat). Diets higher in CHO resulted in shorter sleep onset latencies and diets higher in protein resulted in fewer wake episodes. There was little effect of the high fat diet on markers of sleep quality and quantity (Lindseth et al., 2011). Finally, Grandner et al. (2010) examined the dietary intake (through questionnaires) of 459 postmenopausal women over 7 d. The only significant finding of this study was that fat intake was negatively associated with total sleep time (Grandner et al., 2010).
From the above studies, it appears that diets high in carbohydrate may result in shorter sleep latencies, while diets high in protein may result in improved sleep quality and diets high in fat may negatively influence total sleep time. However, additional research is necessary in this area.
Tryptophan
As mentioned above, the synthesis of 5-HT in the brain is dependent on the availability of its precursor Trp. Further, 5-HT is a precursor to melatonin in the pineal gland (Silber & Schmitt, 2010). There have been numerous studies investigating the effects of tryptophan supplementation on sleep (for review, see Silber & Schmitt, 2010) and it appears that doses of Trp as low as 1 g can improve sleep latency and subjective sleep quality. This can be achieved by consuming ~300 g of turkey or ~200 g of pumpkin seeds.
Melatonin
Melatonin is a hormone that is associated with circadian rhythms (Morin & Benca, 2012) and some research has demonstrated sedative/hypnotic effects of this compound (Buscemi et al., 2005). However, research investigating the use of melatonin for primary insomnia demonstrates inconclusive results (Morin & Benca, 2012). A meta-analysis reported a reduction in sleep onset latency of 7.2 min and concluded that while melatonin appeared safe for short-term use, there was no evidence that melatonin was effective for most primary sleep disorders (Buscemi et al., 2005).
Another recently investigated intervention is tart cherry juice. Tart cherries contain high concentrations of melatonin and when consumed over a 2 wk period improved subjective insomnia symptoms when compared to placebo (Pigeon et al., 2010). There have also been reports of modest improvements in sleep time and quality (Howatson et al., 2011).
Valerian
Valerian is an herb that binds to GABA type A receptors and is thought to induce a general calming effect on the body (Wheatley, 2005). Results of a meta-analysis showed subjective improvement in sleep quality, but not quantity (Fernandez-San-Martin et al., 2010).
Other Nutritional Interventions
Nucleotides are believed to be involved in the physiological function of sleep, in particular uridine monophosphate (5’UMP) and adenosine monophosphate (5’AMP). 5’UMP causes a depressive effect on the central nervous system and one study that administered low doses prior to sleep reported improvements in some sleep indices (Chagoya de Sanchez et al., 1996). 5’AMP has hypnotic properties and levels of this nucleotide decline during wakefulness (Sanchez et al., 2009). 5’AMP acts on the adenosine A2A receptors in the venterolateral nuclei region of the brain, which is believed to be related to insomnia, pain and depression (Cubero et al., 2009). These nucleotides have been studied via investigations regarding the possible hypnotic effects of infant formula (Sanchez et al., 2009). In this study, the sleep-promoting formula contained high levels of L-tryptophan and carbohydrates, low levels of protein, and 5’UMP and 5’AMP. Fifty- four children were monitored over 1 wk using actigraphy, with results showing increased time in bed and increased sleep efficiency. The authors suggested that these results supported the concept of chrononutrition, i.e., the influence of time of day at which food is ingested having effects on different biological rhythms, such as sleep and wakefulness. However, no blood measures were made and thus it was not possible to determine whether the ingested compounds were transported from the digestive system to the bloodstream and which of the ingredients were actively involved in enhancing sleep.
Glycine (a non-essential amino acid) functions as an inhibitory neurotransmitter in the central nervous system and also acts as a co-agonist of glutamate receptors. Glycine has been shown to improve subjective sleep in a recent Japanese study (Bannai et al., 2012). Yamadera et al. (2007) also reported shorter sleep onset latencies measured by polysomnography (“gold standard” for sleep assessment). The authors speculated from previous studies on rodents that potential mechanisms may involve increased vasodilation and thus lowering of core temperature, and increased extracellular serotonin release in the prefrontal cortex of the brain (Yamadera et al., 2007).
L-theanine is an amino acid analogue present in tea but not coffee that demonstrates pharmacological actions such as promoting feelings of calmness and reduced alertness. One study reported that L-theanine partially counteracted the caffeine-induced decrease in slow wave sleep in rats (Jang et al., 2012).
There are also numerous other traditional products that are purported sleep aids including passionflower, kava, St. John’s wort, lysine, magnesium, lavender, skullcap, lemon balm, magnolia bark, 5-HTP and GABA. While the majority of these products have not been adequately investigated in the scientific literature, many can be found in sleep aid supplements that can be purchased over-the-counter in pharmacies and health food suppliers. However, like many available supplements, there is always the danger that these purported sleep aids may contain illegal substances and thus should be used with caution.
PRACTICAL APPLICATIONS
Athletes should focus on utilising good sleep hygiene to maximise sleep (See previous Sports Science Exchange article on “Sleep in Elite Athletes”). While research is minimal and somewhat inconclusive, several practical recommendations may be suggested:
- High glycemic index (GI) foods such as white rice, pasta, bread and potatoes may promote sleep. However, they should be consumed more than one hour prior to bedtime.
- Diets high in carbohydrate may result in shorter sleep latencies.
- Diets high in protein may result in improved sleep quality.
- Diets high in fat may negatively influence total sleep time.
- When total caloric intake is decreased, sleep quality may be disturbed.
- Small doses of tryptophan (1 g) may improve both sleep latency and sleep quality. This can be achieved by consuming ~300 g of turkey or ~200 g of pumpkin seeds.
- The hormone melatonin and foods that have a high melatonin concentration may decrease sleep onset time.
- Subjective sleep quality may be improved with the ingestion of the herb valerian.
SUMMARY
While the quantity of research investigating the effects of nutritional interventions on sleep is increasing, future research needs to highlight the importance of nutritional and dietary interventions to enhance sleep both in the general population and in athletes. Careful examination of both the timing of food ingestion and the use of different interventions would provide invaluable information to athletes on how to improve sleep through nutritional means. Ideally, research will lead to nutritional interventions for optimising both sleep quality and quantity, as well as enhancing athlete recovery from training and competition.
REFERENCES
Afaghi, A., H. O’Connor, and C.M. Chow (2007). High-glycemic-index carbohydrate meals shorten sleep onset. Am. J. Clin. Nutr. 85:426-430.
Afaghi, A., H. O’Connor, and C.M. Chow (2008). Acute effects of the very low carbohydrate diet on sleep indices. Nutr. Neurosi. 11:146-154.
Bannai, M., N. Kawai, K. Ono, K. Nakahara, and N. Murakami (2012). The effects of glycine on subjective daytime performance in partially sleep-restricted healthy volunteers. Front. Neurol. 3: 61.
Buscemi, N., B. Vandermeer, N. Hooton, R. Pandya, L. Tjosvold, L. Hartling, G. Baker, T. P. Klassen, and S. Vohra (2005). The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J. Gen. Intern. Med. 20:1151-1158.
Carskadon, M.A., and W.C. Dement (2011). Normal human sleep: an overview. In: M.H. Kryger, T. Roth and W.C. Dement (eds.) Principles and Practice of Sleep Medicine. St Louis: Elsevier, pp. 16-26.
Chagoya de Sanchez, V., R. Hernandez-Munoz, J. Suarez, S. Vidrio, L. Yanez, R. Aguilar-Roblero, A. Oksenberg, A. Vega-Gonzalez, L. Villalobos, L. Rosenthal, F. Fernandez-Cancino, R. Drucker-Colin, and M. Diaz-Munoz (1996). Temporal variations of adenosine metabolism in human blood. Chronobiol. Int. 13:163-77.
Cubero, J., B. Chanclon, S. Sanchez, M. Rivero, A. B. Rodriguez, and C. Barriga (2009). Improving the quality of infant sleep through the inclusion at supper of cereals enriched with tryptophan, adenosine-5’-phosphate, and uridine-5’- phosphate. Nutr. Neurosci. 12:272-80.
Fernandez-San-Martin, M.I., R. Masa-Font, L. Palacios-Soler, P. Sancho-Gomez, C. Calbo-Caldentey, and G. Flores-Mateo (2010). Effectiveness of Valerian on insomnia: a meta-analysis of randomized placebo-controlled trials. Sleep Med. 11:505-511.
Fernstrom, J.D., and R.J. Wurtman (1971). Brain serotonin content: physiological dependence on plasma tryptophan levels. Science 173:149-152.
Grandner, M.A., D.F. Kripke, N. Naidoo, and R.D. Langer (2010). Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 11:180-184.
Grimmett, A., and M.N. Sillence (2005). Calmatives for the excitable horse: a review of L-tryptophan. Vet. J. 170:24-32.
Hartmann, M.K., A.H. Crisp, G. Evans, M.K. Gaitonde, and B.R. Kirkwood (1979).
Short-term effects of CHO, fat and protein loads on total tryptophan/tyrosine levels in plasma as related to %REM sleep. Waking Sleeping 3:63-68.
Heine, W.E. (1999). The significance of tryptophan in infant nutrition. Adv. Exp. Med. Biol. 467:705-710.
Howatson, G., P.G. Bell, J. Tallent, B. Middleton, M.P. McHugh, and J. Ellis (2011).
Effect of tart cherry juice (Prunus cerasus) on melatonin levels and enhanced sleep quality. Eur. J. Nutr. 51:909-916.
Jalilolghadr, S., A. Afaghi, H. O’Connor, and C.M. Chow (2011). Effect of low and high glycaemic index drink on sleep pattern in children. J. Pak. Med. Assoc. 61:533-536.
Jang, H.S., J.Y. Jung, I.S. Jang, K.H. Jang, S.H. Kim, J.H. Ha, K. Suk, and M.
G. Lee (2012). L-theanine partially counteracts caffeine-induced sleep disturbances in rats. Pharmacol. Biochem. Behav. 101:217-221.
Kwan, R.M., S. Thomas, and M.A. Mir (1986). Effects of a low carbohydrate isoenergetic diet on sleep behavior and pulmonary functions in healthy female adult humans. J. Nutr. 116: 2393-2402.
Lacey, J.H., C. Hawkins, and A.H. Crisp (1978). Effects of dietary protein on sleep E.E.G. in normal subjects. Adv. Biosci. 21:245-247.
Lindseth, G., P. Lindseth, and M. Thompson (2011). Nutritional effects on sleep. West. J. Nurs. Res. 2011 Aug 4. [Epub ahead of print].
Morin, C.M. and R. Benca (2012). Chronic insomnia. Lancet 379:1129-1141.
Orr, W.C., G. Shadid, M.J. Harnish, and S. Elsenbruch (1997). Meal composition and its effect on postprandial sleepiness. Physiol. Behav. 62:709-712.
Pigeon, W.R., M. Carr, C. Gorman, and M.L. Perlis (2010). Effects of a tart cherry juice beverage on the sleep of older adults with insomnia: a pilot study. J. Med. Food 13:579-583.
Porter, J.M., and J.A. Horne (1981). Bed-time food supplements and sleep: effects of different carbohydrate levels. Electroencephalogr. Clin. Neurophysiol. 51:426-433.
Sanchez, C. ., J. Cubero, J. Sanchez, B. Chanclon, M. Rivero, A.B. Rodriguez, and C. Barriga (2009). The possible role of human milk nucleotides as sleep inducers. Nutr. Neurosci. 12:2-8.
Saper, C.B., T.E. Scammell, and J. Lu (2005). Hypothalamic regulation of sleep and circadian rhythms. Nature 437:1257-1263.
Silber, B.Y. and J.A. Schmitt (2010). Effects of tryptophan loading on human cognition, mood, and sleep. Neurosci. Biobehav. Rev. 34:387-407.
Wheatley, D. (2005). Medicinal plants for insomnia: a review of their pharmacology, efficacy and tolerability. J. Psychopharmacol. 19:414-421.
Yamadera, W., K. Inagawa, S. Chiba, M. Bannai, M. Takahashi, and K. Nakayama (2007). Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes. Sleep Biol. Rhythms 5:126-131.
Zammit, G.K., A. Kolevzon, M. Fauci, R. Shindledecker, and S. Ackerman (1995). Postprandial sleep in healthy men. Sleep 18:229-231.