KEY POINTS
-
Regular moderate exercise reduces the risk of infection compared with a sedentary lifestyle, but very prolonged bouts of exercise and periods of intensified training or competition are associated with increased risk of infection. In athletes, a common observation is that symptoms of respiratory illness cluster around competitions and these can impair exercise performance.
-
Prolonged bouts of strenuous exercise have been shown to result in transient depression of white blood cell functions and it is suggested that such changes create an “open window” of decreased host protection, during which viruses and bacteria can gain a foothold, increasing the risk of developing an infection. Other factors such as psychological stress, lack of sleep and malnutrition can also depress immunity and lead to increased risk of infection.
-
Periods of intensified training with insufficient recovery may result in a temporary state of immunodepression which should recover with a few days of relative rest.
-
There are several behavioral, nutritional and training strategies that can be adopted to limit exercise-induced immunodepression and minimise the risk of infection. Athletes can limit their risk of infection by avoiding close contact with people who are showing symptoms of infection, by practising good hand, oral and food hygiene, and by avoiding the sharing of personal items such as towels and drink bottles.
-
For maintaining robust immunity, getting adequate recovery and sleep is important, as is avoiding deficiencies of protein and micronutrients (particularly iron, zinc and vitamins A, D, E, B6 and B12).
-
Athletes are advised to ingest carbohydrate (30-60 g [1-2 oz] per h) during prolonged training sessions, and consume – on a daily basis – plant polyphenol (flavonoid) containing supplements or foodstuffs and Lactobacillus probiotics. Vitamin D3 supplementation may also be desirable for some athletes as vitamin D deficiency is common in the winter months.
INTRODUCTION
The amount of physical activity that a person does influences his/ her risk of infection, most likely by affecting immune function. It is known that regular moderate exercise reduces the risk of infection compared with a sedentary lifestyle (Matthews et al., 2002; Nieman et al., 2011), but very prolonged bouts of exercise and periods of intensified training are associated with increased infection risk. Acute bouts of prolonged strenuous exercise cause a temporary depression of various aspects of immune function that typically last for up to 24 h after exercise (Walsh et al., 2011b). Several studies indicate that the incidence of upper respiratory tract illness symptoms (URS) is increased in the days after prolonged strenuous endurance events (Gleeson et al., 2013; Walsh et al., 2011b) and it has been generally assumed that this reflects the temporary depression of immune function induced by prolonged exercise. Infections can occur following exposure to new pathogens, but can also be caused by reactivation of a latent virus. More recently, however, it has been proposed that at least some of the URS episodes in athletes are attributable to upper airway inflammation rather than to infections with pathogens (Spence et al., 2007). Periods of intensified training lasting a week or more have been shown to chronically depress several aspects of immune function (Gleeson et al., 2013) and although elite athletes are not clinically immune deficient, it is possible that the combined effects of small changes in several immune factors may compromise resistance to common minor illnesses, particularly during periods of prolonged heavy training and at times of major competitions.
EXERCISE, IMMUNITY AND ILLNESS IN ATHLETES
Causes of Illness in Athletes
The most common illnesses in athletes (and the general population) are viral infections of the upper respiratory tract (i.e., the common cold and influenza), which are more common in the winter months. Adults typically experience between two and four episodes of respiratory illness per year. Athletes can also develop similar symptoms (e.g., sore throat) due to allergy or inflammation caused by inhalation of cold, dry or polluted air (Bermon, 2007). These symptoms are generally trivial, but no matter whether the cause is infectious or allergic inflammation, they can cause an athlete to interrupt training, under-perform or even miss an important competition. A recent survey of hundreds of elite Great Britain athletes in 30 different Olympic sports reported that among the reasons for missing training, in 33% of cases it was because of infection (most commonly of the respiratory tract). Analysis of the 126 reported illnesses among 1,851 athletes competing in the 2011 World Athletics Championships in Daegu, South Korea, revealed that 40% of illnesses affected the upper respiratory tract with confirmed infection in about 20% of cases (Alonso et al., 2012). Other main causes of sickness were associated with exercise-induced dehydration (12% of cases) and gastroenteritis/diarrhoea (10% of cases). Similar studies on athletes competing at major events lasting 2-3 wk indicated that typically ~7% of registered athletes suffer an illness episode at this time (Alonso et al., 2010; Engebretsen et al., 2010, 2013). Interestingly, all these studies indicated that the incidence of illness was somewhat higher in female athletes when compared with their male counterparts.
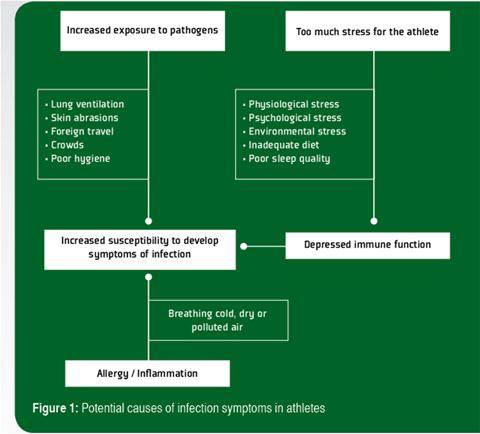
Prolonged bouts of strenuous exercise have been shown to result in transient depression of white blood cell (leukocyte) functions and it is suggested that such changes create an “open window” of decreased host protection, during which viruses and bacteria can gain a foothold, increasing the risk of developing an infection (Walsh et al., 2011b). Other factors such as psychological stress, lack of sleep and inadequate nutrition (particularly deficiencies of protein and essential micronutrients) can also depress immunity (Walsh et al., 2011a) and lead to increased risk of infection. There are also some situations in which an athlete’s exposure to infectious agents may be increased, which is the other important determinant of infection risk. During exercise, exposure of the lungs to airborne bacteria and viruses increases because of the higher rate and depth of breathing. Allergy and inflammation of the airways caused by breathing cold, dry or polluted air are an alternative cause of URS that could be mistaken for a respiratory infection in athletes. An increase in gut permeability may also allow entry of gut bacterial endotoxins into the circulation, particularly during prolonged exercise in the heat. In contact sports, skin abrasions may occur increasing the risk of transdermal infections. In some sports the competitors may be in close proximity to large crowds. Air travel to foreign countries may be involved. Hence, the cause of the increased incidence of infection symptoms in athletes is most likely multifactorial (Figure 1).
Acute Prolonged Exercise Effects on Immune Function
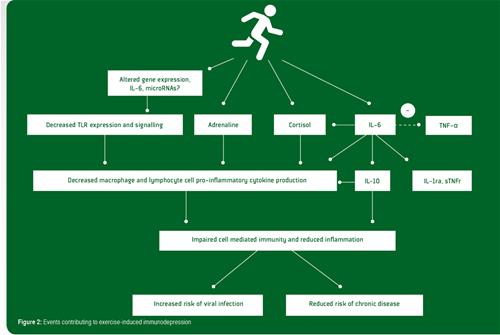
Prolonged bouts of strenuous exercise have a temporary negative impact on immune function. Post-exercise immune function depression is most pronounced when the exercise is continuous, prolonged (>1.5 h), of moderate to high intensity (55-75% of aerobic capacity) and performed without food intake (Gleeson, 2013). Many aspects of innate immunity including neutrophil chemotaxis, phagocytosis, degranulation and oxidative burst activity, monocyte toll-like receptor (TLR) expression and natural killer cell cytotoxic activity are depressed by prolonged exercise. Similarly, several important acquired (specific) immune functions including antigen presentation by monocytes/macrophages, immunoglobulin production by B lymphocytes, T lymphocyte cytokine (e.g., interferon-gamma) production and proliferation are reduced after prolonged exercise. Mucosal immune protection may also be compromised: Although the salivary secretory immunoglobulin A (SIgA) response to acute exercise is variable, very prolonged bouts of exercise (e.g., running a marathon) are commonly reported to result in decreased SIgA secretion (Walsh et al., 2011b). The causes of immune depression after prolonged exercise are thought to be largely due to increases in circulating stress hormones (e.g., epinephrine and cortisol) and alterations in the pro-/anti-inflammatory cytokine balance (particularly elevated circulating levels of interleukin (IL)- 6, IL-10, IL-1 receptor antagonist (IL-1ra) and soluble tumor necrosis factor (TNF) receptors) that have inhibitory actions on immune activation.
Recent studies examining gene expression in leukocytes after prolonged exercise indicate that there is increased expression of many genes involved in anti-inflammatory actions and down-regulation of genes of the TLR receptor signalling pathway that leads to pro-inflammatory cytokine production and immune activation (Abbasi et al., 2013, 2014). Many factors and hormones which are induced by exercise may be involved in organizing this broad anti-inflammatory gene reaction. These factors may include catecholamines, cortisol, growth hormone, heat shock proteins and muscle-derived IL-6 (Gleeson et al., 2011). Cortisol is known for its vast array of immunosuppressive/anti-inflammatory functions and is highly likely to play an important role in this context. It seems, however, safe to say that IL-6 probably is the key player in orchestrating this broad anti-inflammatory reaction. During exercise, IL-6 is released from contracting muscle fibers and causes release of IL-10 and IL-1ra, adrenocorticotrophic hormone and cortisol, as well as acute phase reactants of hepatocytes (e.g., α1 acid glycoprotein and C-reactive protein). The induction of IL-10 production through exercise-induced elevations of circulating IL-6 may represent a direct, preemptive anti-inflammatory event rather than a balancing counter regulation against some primary inflammatory stimulus. Although IL-6 and its followers can explain most of the anti-inflammatory reaction reported, it is possible that additional mechanisms may be at work. For example, exercise results in rapid induction of microRNAs (Tonevitsky et al., 2013) which are capable of interfering with TLRs, and it has been suggested that such a mechanism might also be instrumental in inducing the anti-inflammatory response to exercise (Abbasi et al., 2014). The various events contributing to exercise-induced immunodepression are summarized in Figure 2.
Chronic Exercise Training Effects on Immune Function
Immune function indices in athletes in the true resting state (i.e., at least 24 h after the last exercise bout) are generally not very different from their sedentary counterparts, except when athletes are engaged in periods of intensified training. In this situation, immune function might not fully recover from successive training sessions and some functions can become chronically depressed (Gleeson et al., 2013). Both T and B lymphocyte functions appear to be sensitive to increases in training load in well-trained athletes undertaking a period of intensified training, with decreases in circulating numbers of Type 1 T cells, inhibition of Type 1 T cell cytokine production, reduced T cell proliferative responses and falls in stimulated B cell immunoglobulin synthesis and SIgA reported. However, to date, the only immune variable that has been consistently associated with increased infection incidence is SIgA. Low concentrations of SIgA in athletes or substantial transient falls in SIgA are associated with increased risk of URS episodes (Neville et al., 2008). In contrast, increases in SIgA can occur after a period of regular moderate exercise training in previously sedentary individuals and could, at least in part, contribute to the apparent reduced susceptibility to URS associated with regular moderate exercise (Walsh et al., 2011b). Illness-prone athletes tend to have low SIgA secretion rate and increased in vitro IL-10 production in whole blood cultures exposed to an antigen challenge (Gleeson & Bishop, 2013), which may weaken immune defences against microorganisms. Athletes with low vitamin D status, high training loads and no prior infection with cytomegalovirus and Epstein-Barr virus also appear to be more susceptible to URS episodes (He et al., 2013).
The prevention of infection is an important research area both in terms of the health of the general population and particularly for athletes undertaking prolonged periods of heavy training. In terms of negative impact on training, repeated periods of infection are akin to recurrent physical injuries that can be catastrophic when they occur as athletes approach major competitions. Therefore, the study by Neville et al. (2008) is particularly encouraging because it showed a retrospective analyses of the salivary samples of 38 Americas Cup athletes taken over 50 wk that when their relative SIgA values fell by 40% or more they were likely to experience infections within 1-2 wk. With the impending availability of rapid “in the field” salivary analysis using hand-held devices, these measurements may offer a way of informing coaches when athletes are most vulnerable to infection and problems associated with increased training loads might be avoided.
A common perception is that exposure to cold wet weather can increase the likelihood of catching the common cold, but the available evidence does not indicate that athletes training and competing in cold conditions experience a greater reduction in immune function compared with thermoneutral conditions (Walsh et al., 2011b). The inhalation of cold dry air can reduce upper airway ciliary movement and decrease mucous flow but it is not clear if athletes who regularly train and compete in cold conditions experience more frequent, more severe or longer-lasting infections. Other environmental extremes (e.g., heat and altitude) do not seem to have a marked impact on immune responses to exercise (Walsh et al., 2011b).
PRACTICAL APPLICATIONS
Guidelines for Maintenance of Immune Health and Limiting the Risk of Infection
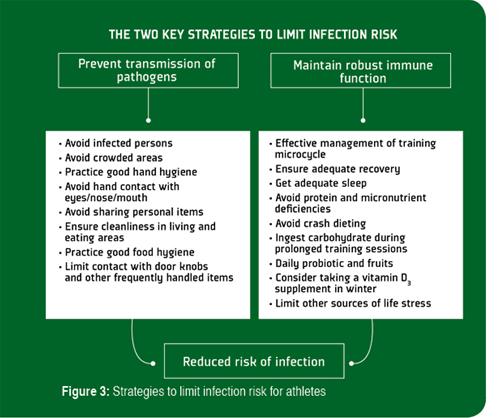
It is generally agreed that prevention is always preferable to treatment, and although there is no single method that completely eliminates the risk of contracting an infection, there are several effective behavioral, nutritional and training strategies (Figure 3) that can limit the extent of exercise-induced immunodepression, lower exposure to pathogens and reduce the risk of infection (Walsh et al., 2011a).
Limiting the Transmission of Infections
The most important guidelines to limit transmission of infections among athletes are good hand hygiene and avoiding contact with persons that are infected (Table 1). Hand washing (with the correct technique to ensure all parts of hands are cleaned effectively) with soap and water is effective against most pathogens, but does not provide continuous protection. Hand gels containing >60% alcohol disinfect effectively, but the protection they provide does not last more than a few minutes, so they need to be applied frequently and this can cause skin drying and irritation. Other sanitization methods include the use of non-alcohol based antimicrobial hand foams that contain cationic biocides and hydrophobic polymers which are claimed to disinfect hands for up to 6 h. However, individuals need to be aware that these products are removed by hand washing and excessive sweating and need to be reapplied every few hours.


Maintaining Robust Immunity and Limiting Training Stress
The other things that athletes can do to limit risk of infection are to adhere to practical guidelines to maintain robust immunity and limit the impact of training stress (Table 2). These guidelines relate mostly to nutritional, training and recovery strategies, and are based on the findings of numerous research studies. The most effective nutritional strategies to maintain robust immune function during intensive training are to avoid deficiencies of essential micronutrients, ingest carbohydrate during exercise and ingest Lactobacillus probiotics on a daily basis. While not all probiotics have been shown to help maintain healthy levels of salivary SIgA, prolonged ingestion of some Lactobacillus strains have provided encouraging results (Gleeson et al., 2012). Therefore, athletes should be advised on how best to fortify their diets with the appropriate type of probiotic. Some studies also suggest that regular consumption of fruits and plant polyphenol supplements (e.g., quercetin) or foodstuffs (e.g., non-alcoholic beer and green tea) can also reduce URS incidence. Many other nutrition supplements, including β-glucan, colostrum, echinacea, glutamine and others claim immune-boosting properties, but there is no compelling scientific evidence that they effectively prevent exercise-induced immune depression.
In addition to obeying the rules of good personal hygiene, the composition of the diet and timing of food intake may also help provide protection against infections. Since immune function is compromised after heavy training and competition, and carbohydrate, protein and fluid ingestion helps restore function (Costa et al., 2012; Fortes et al., 2012; Witard et al., 2014), it is important that athletes are encouraged to develop feeding strategies that focus on the post-exercise period as part of their overall nutritional plans.
SUMMARY
There is now substantial evidence to support the notion that prolonged strenuous exercise is associated with a transient suppression of immune functions which usually recover within 24 h. However, in situations of intensive training a lack of sufficient recovery between exercise sessions can lead to chronic depression of immune responses. It has been suggested that such effects on host defence account for the higher incidence of URS among highly trained athletes, leading to absence from training and impaired performance. While it is certainly true that URS are commonly reported in athletes, an infectious cause of these symptoms has not always been confirmed. There are various training, behavioral and nutritional strategies that can help to minimise URS risk and these should become part of the athlete’s normal routine.
REFERENCES
Abbasi, A., E. Fehrenbach, M. Hauth, M. Walter, J. Hudemann, V. Wank, A.M. Niess, and H. Northoff (2013). Changes in spontaneous and LPS-induced ex vivo cytokine production and mRNA expression in male and female athletes following prolong exhaustive exercise. Exerc. Immunol. Rev. 19:8-28.
Abbasi, A., M. Hauth, M. Walter, J. Hudemann, V. Wank, A.M. Niess, and H. Northoff (2014). Exhaustive exercise modifies different gene expression profiles and pathways in LPS-stimulated and un-stimulated whole blood cultures. Brain Behav. Immun. 39:130-141.
Alonso, J.M., P.M. Tscholl, L. Engebretsen, M. Mountjoy, J. Dvorak, and A. Junge (2010). Occurrence of injuries and illnesses during the 2009 IAAF World Athletics Championships. Br. J. Sports Med. 44:1100-1105.
Alonso, J.M., P. Edouard, G. Fischetto, B. Adams, F. Depiesse, and M. Mountjoy (2012). Determination of future prevention strategies in elite track and field: analysis of Daegu 2011 IAAF Championships injuries and illnesses surveillance. Br. J. Sports Med. 46:505-514.
Bermon, S. (2007). Airway inflammation and upper respiratory tract infection in athletes: is there a link? Exerc. Immunol. Rev. 13:6-14.
Costa, R.J., M.B. Fortes, K. Richardson, J.L. Bilzon, and N.P. Walsh (2012). The effects of postexercise feeding on saliva antimicrobial proteins. Int. J. Sport Nutr. Exerc. Metab. 22:184-191.
Engebretsen, L., K. Steffen, J.M. Alonso, M. Aubry, J. Dvorak, A. Junge, W. Meeuwisse, M. Mountjoy, P. Renström, and M. Wilkinson (2010). Sports injuries and illnesses during the Winter Olympic Games 2010. Br. J. Sports Med. 44:772-780.
Engebretsen, L., T. Soligard, K. Steffen, J.M. Alonso, M. Aubry, R. Budgett, J. Dvorak, M. Jegathesan, W.H. Meeuwisse, M. Mountjoy, D. Palmer-Green, I. Vanhegan, and P.A. Renström (2013). Sports injuries and illnesses during the London Summer Olympic Games 2012. Br. J. Sports Med. 47:407-414.
Fortes, M.B., B.C. Diment, U. Di Felice, and N.P. Walsh (2012). Dehydration decreases saliva antimicrobial proteins important for mucosal immunity. Appl. Physiol. Nutr. Metab. 37:850-859.
Gleeson, M., N.C. Bishop, D.J. Stensel, M.R. Lindley, S.S. Mastana, and M.A. Nimmo (2011). The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 11:607-615.
Gleeson, M., J. Siegler, L.M. Burke, S. Stear, and L.M. Castell (2012). A to Z of nutritional supplements: Dietary supplements, sports nutrition foods and ergogenic aids for health and performance – Part 31. (Probiotics). Br. J. Sports Med. 46:377-378.
Gleeson, M. (2013). Exercise, nutrition and immunity. In: P.C. Calder and P. Yaqoob (eds.), Diet, Immunity and Inflammation. Cambridge: Woodhead Publishing, pp. 652-685.
Gleeson, M., and N.C. Bishop (2013). URI in athletes: Are mucosal immunity and cytokine responses key risk factors? Exerc. Sport Sci. Rev. 41:148-153.
Gleeson, M., N.C. Bishop, and N.P. Walsh (eds.) (2013). Exercise Immunology. Abingdon: Routledge.
He, C.-S., M. Handzlik, W.D. Fraser, A. Muhamad, H. Preston, A. Richardson, and M. Gleeson (2013). Influence of vitamin D status on respiratory infection incidence and immune function during 4 months of winter training in endurance sport athletes. Exerc. Immunol. Rev. 19:86-101.
Larson-Meyer D.E., and K.S. Willis (2010). Vitamin D and athletes. Curr. Sports Med. Rep. 9:220-226.
Matthews, C.E., I.S. Ockene, P.S. Freedson, M.C. Rosal, P.A. Merriam, and J.R. Hebert (2002). Moderate to vigorous physical activity and the risk of upper-respiratory tract infection. Med. Sci. Sports Exerc. 34:1242-1248.
Neville, V., M. Gleeson, and J.P. Folland (2008). Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes. Med. Sci. Sports Exerc. 40:1228- 1236.
Nieman, D.C., D.A. Henson, M.D. Austin, and W. Sha (2011). Upper respiratory tract infection is reduced in physically fit and active adults. Br. J. Sports Med. 45:987-992.
Robson-Ansley, P., G. Howatson, J. Tallent, K. Mitcheson, I. Walshe, C. Toms, G. Du Toit, M. Smith, and L. Ansley (2012). Prevalence of allergy and upper respiratory tract symptoms in runners of the London Marathon. Med. Sci. Sports Exerc. 44:999- 1004.
Spence, L., W.J. Brown, D.B. Pyne, M.D. Nissen, T.P. Sloots, J.G. McCormack, A.S. Locke, and P.A. Fricker (2007). Incidence, etiology, and symptomatology of upper respiratory illness in elite athletes. Med. Sci. Sports Exerc. 39:577-586.
Tonevitsky, A.G., D.V. Maltseva, A. Abbasi, T.R. Samatov, D.A. Sakharov, M.U. Shkurnikov, A.E. Lebedev, V.V. Galatenko, A.I. Grigoriev, and H. Northoff (2013). Dynamically regulated miRNA-mRNA networks revealed by exercise. BMC Physiol. 13:9
Walsh. N.P., M. Gleeson, D.B. Pyne, D.C. Nieman, F.S. Dhabhar, R.J. Shephard, S.J. Oliver, S. Bermon, and A. Kajėnienė (2011a). Position Statement Part Two: Maintaining immune health. Exerc. Immunol. Rev. 17:64-103.
Walsh, N.P., M. Gleeson, R.J. Shephard, M. Gleeson, J.A. Woods, N.C.Bishop, M. Fleshner, C. Green, B.K. Pedersen, L. Hoffman-Goetz, C.J. Rogers, H. Northoff, A. Abbasi, and P. Simon (2011b). Position Statement Part One: Immune function and exercise. Exerc. Immunol. Rev. 17:6-63.
Witard, O.C., J.E. Turner, S.R. Jackman, A.K. Kies, A.E. Jeukendrup, J.A. Bosch, and K.D. Tipton (2014). High dietary protein restores overreaching induced impairments in leukocyte trafficking and reduces the incidence of upper respiratory tract infection in elite cyclists. Brain. Behav. Immun. 44:1689-1697.