KEY POINTS
- Aging is associated with the progressive loss of skeletal muscle mass and decline in physical function, which may result in reduced mobility and subsequent loss of independence.
- Exercise is a potent stimulator of muscle protein synthesis in older adults.
- Dietary practices by athletes to augment performance and recovery may be relevant to the preservation of skeletal muscle mass and strength in older adults.
- Older adults should aim to consume 0.3 to 0.4 g/kg of protein per eating occasion for a total of 1.2 to 1.6 g/kg/day of protein to accompany exercise regimens.
- Dietary supplementation commonly utilized by athletes, such as creatine, long chain n-3 polyunsaturated fatty acids (n-3 PUFAs), and inorganic nitrate, may improve skeletal muscle health in older adults.
- Future studies are required to better understand the impact of ingesting multiple ingredient dietary supplements on both skeletal muscle health and cognition in older adults.
INTRODUCTION
The aging population is growing rapidly. By the year 2050, 1 in every 6 individuals will be over the age of 65 (World population ageing 2019, 2020). Aging is accompanied by the progressive loss of skeletal muscle mass (sarcopenia) and strength. With typical sarcopenic decline, skeletal muscle mass is lost at a rate of ~1% per year (Janssen et al., 2002) and muscle strength (e.g., 1-repetition maximum) at ~3% per year (Reid et al., 2014). Therefore, skeletal muscle retention with advancing age is of incredible physiological importance, as the loss of skeletal muscle strength compromises physical independence and ability to perform activities of daily living (Maresova et al., 2019). To this end, strategies to augment or maintain skeletal muscle mass and its functional capacity are a primary consideration in preserving the quality of life of older adults.
Optimizing skeletal muscle mass and physical function are also primary objectives for athletes in the pursuit of elite performance. Athletes frequently adopt specific training and nutrition regimens to enhance skeletal muscle remodeling and elicit hypertrophy. Despite athletic intervention strategies being performance focused, many of the nutritional and exercise principles utilized by athletes are directly applicable to improving skeletal muscle health in older adults. Although both athletic and older populations can accumulate training hours to achieve competitive goals, the primary objectives of an athlete are to benefit performance, whereas for general healthy older populations, physical activity provides a means for maintaining independence, reducing the risk of falls, and establishing or continuing social interaction (Bertera, 2003). Consequently, the nutrition intervention strategies discussed in this review should be applied in the context of maximizing the benefits of physical activity and, as such, to support more active aging. This Sports Science Exchange (SSE) article will discuss sports nutrition strategies used by athletes and the potential application in a healthy aging population with a focus on skeletal muscle mass and physical function (Oikawa et al., 2021).
Exercise is associated with numerous health outcomes, including the preservation of muscle mass and function (Ruegsegger &Booth, 2018). integrative regulatory and often redundant pathways have evolved to detect and respond to human movement, here we consider the complex challenges of designing a pill that might mimic the extensive range of exercise benefits.
DIETARY PROTEIN
The Institute of Medicine (2005) recommends adults consume 0.8 g of protein/kg per day. However, the total daily protein requirements for athletes are suggested to be higher (1.2 - 2.0 g/kg/d) to support the repair, replacement, and remodeling of damaged proteins and to optimize the reconditioning of various tissues (muscle, bone, and connective tissues) following exercise (Thomas et al., 2016). Further, a meta-analysis highlighted the benefits of increasing daily protein intake on lean body mass (LBM) during training in both young and older adults (Cermak et al., 2012). Additionally, protein ingestion may also facilitate adaptation to endurance exercise training (Churchward-Venne et al., 2020). Greater improvements in maximal oxygen uptake (V̇O2max) were observed following the daily ingestion of casein protein with training compared with a placebo isocaloric group (Knuiman et al., 2019). Importantly, exercise and protein ingestion act together to stimulate muscle protein synthesis (MPS) (Biolo et al., 1997). When protein is consumed after exercise, the two act synergistically to increase MPS to a greater extent than either stimulus independently (Biolo et al., 1997). Thus, combining exercise with protein feedings represents an effective strategy for muscle preservation/growth in both young and older people (Phillips et al., 2009).
Resistance and aerobic exercise increase MPS in older people (Bell et al., 2015) and the engagement in life long physical activity attenuates the loss of LBM with age (Zampieri et al., 2015). Importantly, individuals who partake in habitual physical activity have superior outcomes of physical function compared with sedentary counterparts (Brach et al., 2004). However, the skeletal muscle of older people can develop a blunted sensitivity to both exercise and protein ingestion, termed anabolic resistance, where a greater volume of exercise and greater amount of protein are required to achieve the same muscle building responses seen in younger adults (Moore et al., 2015). Thus, nutritional strategies to optimize the benefits of physical activity are imperative.
Athletes are recommended to ingest ~20 g of high-quality protein following exercise to enhance MPS (Moore et al., 2009). However, 20 g of protein may be insufficient to maximize MPS in older adults, both acutely (Yang et al., 2012) and chronically, when combined with resistance exercise (Atherton et al., 2020). Indeed, Moore et al. (2015) found that older adults require ~40% more dietary protein after exercise to maximize MPS, compared with the amount required by younger adults. This equates to a serving of 35 g (~0.4 g/kg/serving) of protein to maximize rates of MPS (assuming a male over 60 years, weighing 88 kg (194 lbs)). Thus, older adults may need to consume greater quantities of daily protein (1.2-1.6 g/kg/d) (Bauer et al., 2019) to maximize rates of MPS and support skeletal muscle anabolism. For more information regarding optimal protein amounts, timing, and type in older adults, see van Loon, 2017.
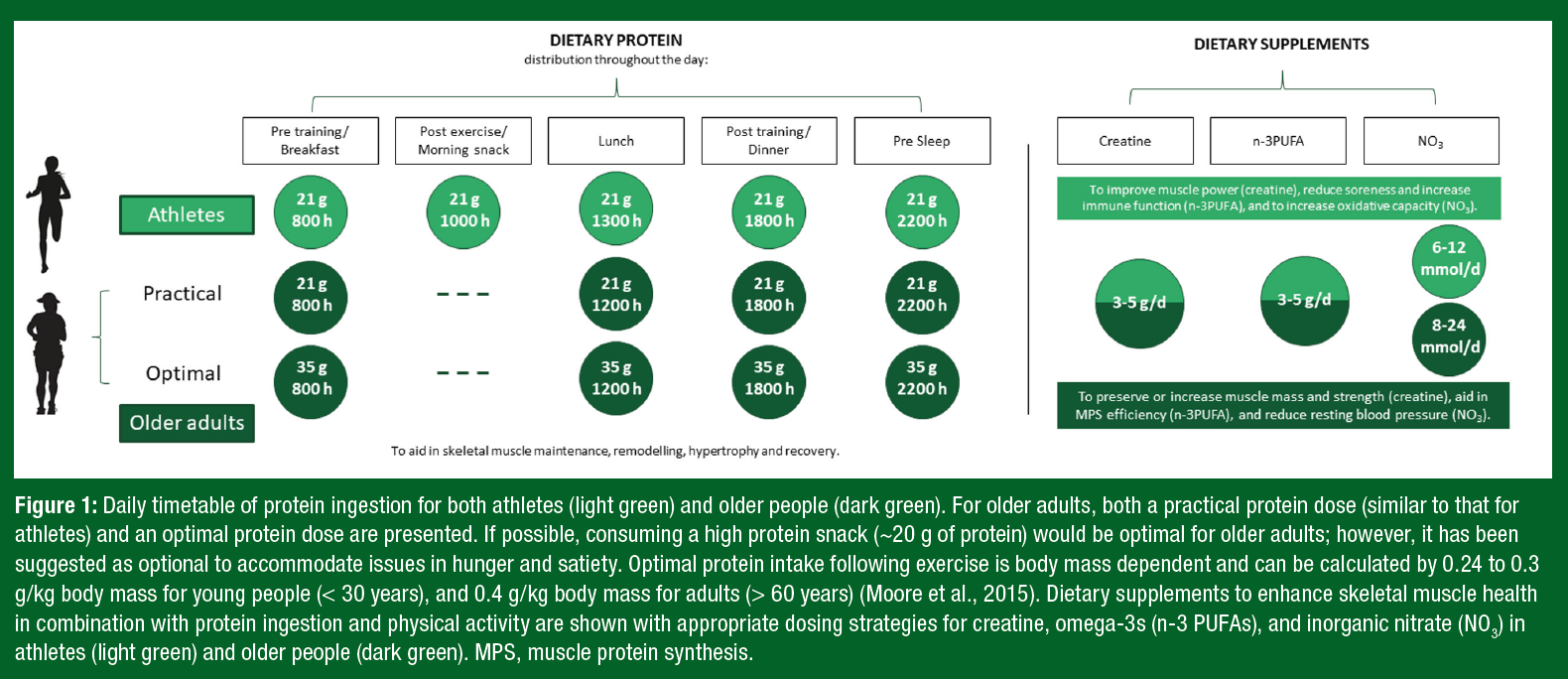
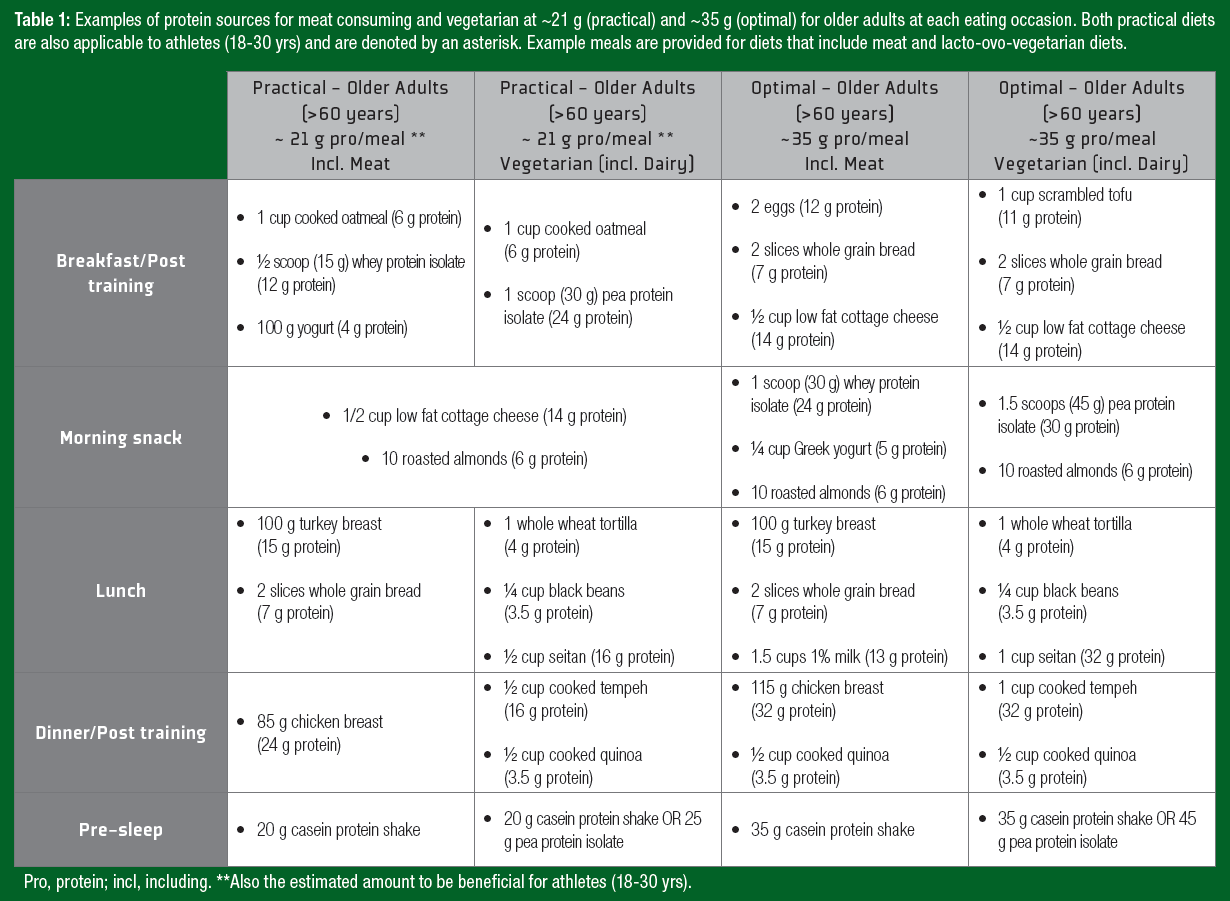
Notably, ~35 g of protein can be a daunting quantity of protein for an older adult to ingest at a single meal. Therefore, despite not being “optimal,” consuming high-quality protein in amounts similar to those of athlete recommendations (~20 g protein/serving) may offer a more practical approach and still contribute to the augmented daily protein recommendations for older adults. A daily timetable of protein ingestion for both athletes and older adults is displayed in Figure 1, with examples of protein-containing meals at the suggested amounts in Table 1.Y
OTHER NUTRIENTS AND DIETARY SUPPLEMENTS
Importantly, the beneficial interaction of exercise and nutrition is not exclusive to protein. Other nutrients and dietary supplements have been applied to augment LBM and strength to enhance physical performance in athletes. These nutritional strategies may also positively impact skeletal muscle health in older people.
Creatine
Creatine monohydrate is a popular dietary supplement among athletes due to its ergogenic ability to enhance the benefits of resistance exercise and to optimize elite performance (Kreider et al., 2017; Terjung et al., 2000). Oral creatine monohydrate supplementation can improve high-intensity exercise capacity by 10% to 20% through increasing skeletal muscle stores of phosphocreatine (PCr) (Kreider et al., 2017). With an increase in the stores of skeletal muscle PCr, individuals can achieve higher workloads during repetitive high-intensity exercise, often resulting in increases in muscle mass and strength (Kreider et al., 2017). Therefore, supplementation with creatine may be of added benefit for athletes competing in sports requiring maximal strength, rapid bursts of power, or intermittent sprints (Kreider, 2003) (Figure 1).
Higher concentrations of muscle PCr have been associated with greater muscle volume and greater peak knee extensor power in older adults, while, conversely, sarcopenic older adults have demonstrated lower content of muscle PCr (Hinkley et al., 2020). Encouragingly, creatine supplementation (3-5 g/d), when combined with resistance training, augmented gains in strength and LBM in older adults (Candow et al., 2015). A meta-analysis of older participants (50-72 years) engaged in exercise training found that individuals consuming creatine had greater increases in LBM and strength compared with participants consuming a placebo (Chilibeck et al., 2017). Importantly, in over 700 participants, no adverse events related to kidney or liver function were reported following prolonged creatine supplementation (Chilibeck et al., 2017).
Long Chain Omega-3 Polyunsaturated Fatty Acids
Athletes consume long chain omega-3 polyunsaturated fatty acids (n-3 PUFAs) supplements to potentially reduce inflammation, improve recovery (following injury), enhance immunity, and, in some cases, increase skeletal muscle metabolic efficiency (Philpott et al., 2019). The incorporation of n-3 PUFAs into the skeletal muscle membrane may improve the transport of nutrients, such as amino acids, into muscle, increasing rates of MPS (Philpott et al., 2019).
In healthy older people, prolonged supplementation with n-3 PUFAs has been shown to increase rates of MPS and therefore may help preserve or facilitate increases in muscle mass with age (Smith et al., 2015). For example, in older men and women, the ingestion of 4 g of n-3 PUFAs for 6 months was associated with increased thigh muscle volume, hand-grip strength, and 1-RM muscle strength compared with those given placebo oil (Smith et al., 2015). Indeed, the ability for n-3 PUFAs to potentiate the MPS response to protein ingestion is compelling given that master athletes (older adults who engage in regular exercise training) still exhibit age-related declines in MPS (Murphy & McGlory, 2021); thus the utility for the use of n-3 PUFAs may be promising for older adults participating in varying levels of physical activity.
Importantly, n-3 PUFAs supplementation has been shown to improve measures of muscle strength in older women but not in older men (Da Boit et al., 2017), and n-3 PUFA supplementation was also found to increase strength and physical performance in a cohort of older women (Rodacki et al., 2012). In a separate study, however, no differences were found in LBM or strength in older men consuming n-3 PUFAs compared with men given a placebo (Cornish et al., 2018). Thus, there may be sex-based differences in the efficacy of supplemental n-3 PUFA to impact skeletal muscle.
Current population intake recommendations for n-3 PUFA are 250 to 500 mg of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) per day; however, given the current evidence, it is recommended that older adults aim to consume 3 to 5 g/d of n-3 PUFA to benefit skeletal muscle metabolism (Figure 1). Future research should aim to provide both insight into the mechanisms driving favorable skeletal muscle health outcomes and to better understand potential sex-based differences with n-3 PUFA supplementation in older adults.
Inorganic Nitrate
Athletes ingest dietary sources of inorganic nitrate (NO3) (e.g., beetroot juice) due to its potential impact on aerobic exercise performance (Jones, 2022). Beetroot or inorganic nitrate supplementation has been shown to benefit recreationally trained and moderately trained men (Jones et al., 2018) but not women (Wickham et al., 2019). However, the effects of NO3 on performance in females has been substantially understudied compared with that in males (Wickham & Spriet, 2019). Consumption of NO3 may improve exercise performance through the restoration of inorganic nitrate cycling, resulting in an augmented vasodilatory response above exercise alone (Jones et al., 2018). Consumption of NO3 on exercise performance utilize a wide array of dosing strategies and amounts. Most commonly, dosages between 6 and 12 mmol/d in young adults have been reported to improve exercise performance (Figure 1). Importantly, the enhanced vascular function effects of NO3 extend beyond the scope of sport; thus supplementation may provide benefits beyond exercise performance.
Diets rich in NO3 are associated with lower blood pressure due to improvements in endothelial mediated vasodilation and increased nitric oxide availability (Matz et al., 2000). Older age is associated with arterial stiffening, impaired vasodilation, and endothelial dysfunction caused, in part, by disruptions in the nitric oxide pathway responsible for maintaining vascular homeostasis (Matz et al., 2000). NO3 ingestion (8-24 mmol/d) has been successful as both a lone intervention as well as complementary therapy to improve vasodilation and lower blood pressure in older adults (Bahadoran et al., 2017) (Figure 1). Of note, increased vasodilatory capacity may result in increased delivery of amino acids to skeletal muscle, augmenting anabolic sensitivity and potentially aiding in the maintenance of skeletal muscle mass over time (Phillips et al., 2012).
Carbohydrate Periodization
The daily intake of carbohydrate and carbohydrate intake during exercise can be adjusted to match the demands and objectives of exercise (Impey et al., 2016). Athletes ingest carbohydrate during training and competition for performance benefits (Williams & Rollo, 2015). Carbohydrate intake may positively influence performance by reducing the perceived effort, as well as supplying substrate for muscle contraction and brain function (Rollo et al., 2020). Athletes modify their carbohydrate intake to match their training objectives and for body composition management.
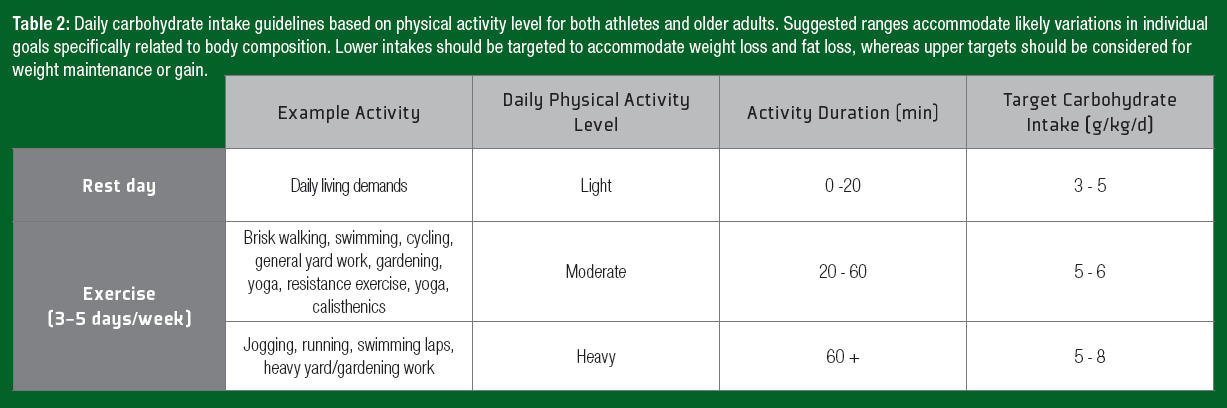
Physical activity is the primary driver to achieve health-related outcomes and maintain physical capabilities in the older adult population. Although performance is often not a primary concern, the ingestion of carbohydrate during exercise may improve the subjective experience by lowering the perception of effort in the healthy aging population (Backhouse et al., 2007). Increasing the adherence to exercise regimens is a key consideration in exercise prescription. Conversely, during days with no structured exercise, the main determinant of energy expenditure is the amount of fat-free mass. The inability to match declining energy requirements with a reduced energy intake will lead to body fat accumulation. In older adults, an increase in body fat and abdominal obesity are associated with increased incidence of non-insulin-dependent diabetes mellitus (Sharda et al., 2015). Thus, recommended modification to daily carbohydrate intake to match daily energy requirements, applicable to both athletes and older adults, are presented in Table 2.
FUTURE DIRECTIONS
Many factors impact the rate of sarcopenia and dynapenia (age-associated loss of muscle strength) in aging adults. Stimuli such as physical inactivity and presence of chronic conditions exert greater influence on the loss of muscle mass and strength than alterations in nutrition in healthy individuals. Nevertheless, optimizing nutrition that may augment or attenuate the decline in skeletal muscle health with aging, particularly when combined with exercise, should be considered when tailoring nutritional strategies to older people. Further, the potential to combine dietary protein with the aforementioned nutritional supplements to enhance muscle protein anabolism is promising and can be undertaken with little risk of harm. It is, however, important to note that the optimal dosing and timing of nutrition strategies specific to older adults discussed above are still to be determined and clearly described.
Though this SSE focused on the benefits of physical activity and nutrition to enhance muscle health and quality of life in older adults, preserving cognitive function or attenuating cognitive decline is also crucial for independent living in this population (Diem et al., 2018). Indeed, dietary intake can modulate cognitive function in aging. For example, high consumption of antioxidants and poly- and mono-unsaturated fats are associated with positive effects on cognitive health outcomes in older adults (Scarmeas et al., 2018). The ingredients discussed in this article were selected based on their potential to enhance skeletal muscle anabolism in athletes and older adults. However, ingestion of these dietary compounds is not exclusive to benefiting skeletal muscle but to the whole body via the systemic circulation. Further, each ingredient discussed has also shown some benefit for cognitive health (Kulzow et al., 2016; Turner et al., 2015; Wightman et al., 2015) albeit in a limited capacity. As such, future research may determine the influence of these nutrients with or without an exercise training regimen on both skeletal muscle and brain health in aging populations.
PRACTICAL RECOMMENDATIONS
- Older adults should aim to consume ~20 to 30 g (0.25-0.4 g/kg) of protein per eating occasion to increase total daily protein intake and maximal post-prandial muscle protein synthesis rates.
- Increases in protein and supplements to augment skeletal muscle health should be considered within the context of a healthy active lifestyle including resistance exercise.
- Supplementation with creatine monohydrate (3-5 g/d), n-3 PUFAs (3-5 g/d), and inorganic nitrate (8-24 mmol/d) may support the improvements in skeletal muscle health following exercise training in older adults.
- Augmented physical fitness is associated with higher levels of cognitive function in older people. Consuming creatine monohydrate, n-3PUFAs, and inorganic nitrate may support such improvements in cognitive function in older adults, but further study is required.
SUMMARY
In summary, healthy older adults may benefit from nutritional strategies used by athletes in pursuit of improved performance. Nutrition interventions discussed in this article (protein, creatine, n-3 PUFA, NO3) do not counteract sarcopenia. However, dietary interventions may augment the benefits of exercise training in the pursuit of more active, healthy aging. Further research is required to determine the mechanisms by which nutrients in isolation or in combination may promote favorable changes in skeletal muscle and cognitive function to maximize the benefits of increasing physical activity levels.
Sara Y. Oikawa, Tristin. D. Brisbois, and Ian Rollo are employed by PepsiCo R&D. The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc. Luc van Loon has received research grants, consulting fees, speaking honoraria, or a combination of these for research on the impact of exercise and nutrition on muscle metabolism; a full overview is provided here: https://www.maastrichtuniversity.nl/l.vanloon
REFERENCES
Atherton, C., L.R. McNaughton, G.L. Close, and A. Sparks (2020). Post-exercise provision of 40 g of protein during whole body resistance training further augments strength adaptations in elderly males. Res. Sports Med. 28:469-483.
Backhouse, S.H., A. Ali, S.J. Biddle, and C. Williams (2007). Carbohydrate ingestion during prolonged high-intensity intermittent exercise: impact on affect and perceived exertion. Scand. J. Med. Sci. Sports 17:605-610.
Bahadoran, Z., P. Mirmiran, A. Kabir, F. Azizi, and A. Ghasemi (2017). The nitrate-independent blood pressure-lowering effect of beetroot juice: A systematic review and meta-analysis. Adv. Nutr. 8:830-838.
Bauer, J., J.E. Morley, A. Schols, L. Ferrucci, A.J. Cruz-Jentoft, E. Dent, E., V.E. Baracos, J.A. Crawford, W. Doehner, S.B. Heymsfield, A. Jatoi, K. Kalantar-Zadeh, M. Lainscak, F. Landi, A. Laviano, M. Mancuso, M. Muscaritoli, C.M. Prado, F. Strasser, S. von Haehling, A.J.S. Coats, and S.D. Anker (2019). Sarcopenia: A time for action. An SCWD position paper. J. Cachexia Sarcopenia Muscle 10:956-961.
Bell, K.E., C. Seguin, G. Parise, S.K. Baker, and S.M. Phillips (2015). Day-to-day changes in muscle protein synthesis in recovery from resistance, aerobic, and high-intensity interval exercise in older men. J. Gerontol. A Biol. Sci. Med. Sci. 70:1024-1029.
Bertera, E.M. (2003). Physical activity and social network contacts in community dwelling older adults. Act. Adapt. Aging 27:113-127.
Biolo, G., K.D. Tipton, S. Klein, and R.R. Wolfe (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am. J. Physiol. 273:E122-E129.
Brach, J.S., E.M. Simonsick, S.B. Kritchevsky, K. Yaffe, and A.B. Newman. Health, Aging and Body Composition Study Research Group (2004). The association between physical function and lifestyle activity and exercise in the health, aging and body composition study. J. Am. Geriat. Soc. 52:502-509.
Candow, D.G., E. Vogt, S. Johannsmeyer, S.C. Forbes, and J.P. Farthing (2015). Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 40:689-694.
Cermak, N.M., P.T. Res, L.C. de Groot, W.H. Saris, and L.J. van Loon (2012). Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: A meta-analysis. Am. J. Clin. Nutr. 96:1454-1464.
Chilibeck, P.D., M. Kaviani, D.G. Candow, and G.A. Zello (2017). Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 8:213-226.
Churchward-Venne, T.A., P.J.M. Pinckaers, J.S.J. Smeets, M.W. Betz, J.M. Senden, J.P.B. Goessens, A.P. Gijsen, I Rollo, L.B. Verdijk, and L.J.C. van Loon (2020). Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: a double-blind randomized trial. Am. J. Clin. Nutr. 112:303-317.
Cornish, S.M., S.B. Myrie, E.M. Bugera, J.E. Chase, D. Turczyn, and M. Pinder (2018). Omega-3 supplementation with resistance training does not improve body composition or lower biomarkers of inflammation more so than resistance training alone in older men. Nutr. Res. 60:87-95.
Da Boit, M., R. Gibson, S. Sivasubramaniam, J.R. Meakin, C.A. Greig, R.M. Aspden, F. Thies, S. Jeromson, D.L. Hamilton, J.R. Speakman, C. Hambly, A.A. Mangoni, T. Preston, and S.R. Gray (2017). Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am. J. Clin. Nutr. 105:151-158.
Diem, S.J., L.Y. Lui, L. Langsetmo, B. Taylor, P.M. Cawthon, J.A. Cauley, K.E. Ensrud. Study of Osteoporotic Fractures (SOF) Research Group. (2018). Effects of mobility and cognition on maintenance of independence and survival among women in late life. J. Gerontol. A Biol. Sci. Med. Sci. 73:1251-1257.
Hinkley, J.M., H.H. Cornnell, R.A. Standley, E.Y. Chen, N.R. Narain, B.P. Greenwood, V. Bussberg, V.V. Tolstikov, M.A. Kiebish, F. Yi, R.B. Vega, B.H. Goodpaster, and P.M. Coen (2020). Older adults with sarcopenia have distinct skeletal muscle phosphodiester, phosphocreatine, and phospholipid profiles. Aging Cell 19:e13135.
Impey, S.G., K.M. Hammond, S.O. Shepherd, A.P. Sharples, C. Stewart, M. Limb, K. Smith, A. Philp, S. Jeromson, D.L. Hamilton, G.L. Close, and J.P. Morton (2016). Fuel for the work required: a practical approach to amalgamating train-low paradigms for endurance athletes. Physiol. Rep. 4:e12803.
Institute of Medicine (2005). Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids T.N.A. Press.
Janssen, I., S.B. Heymsfield, and R. Ross (2002). Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 50:889-896.
Jones, A.M. (2022). Dietary nitrate and exercise performance: New strings to the beetroot bow. SSE #222.
Jones, A.M., C. Thompson, L.J. Wylie, and A. Vanhatalo (2018). Dietary nitrate and physical performance. Annu. Rev. Nutr, 38:303-328.
Knuiman, P., L.J. van Loon, J. Wouters, M. Hopman, and M. Mensink (2019). Protein supplementation elicits greater gains in maximal oxygen uptake capacity and stimulates lean mass accretion during prolonged endurance training: a double-blind randomized controlled trial. Am. J. Clin. Nutr. 110:508-518.
Kreider, R.B. (2003). Effects of creatine supplementation on performance and training adaptations. Mol. Cell. Biochem. 244:88-94.
Kreider, R.B., D.S. Kalman, J. Antonio, T.N. Ziegenfuss, R. Wildman, R. Collins, D.G. Candow, S.M. Kleiner, A.L. Almada, and H.L. Lopez (2017). International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 14:18.
Kulzow, N., A.V. Witte, L. Kerti, U. Grittner, J.P. Schuchardt, A. Hahn, and A. Floel (2016). Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. J. Alzheim. Dis. 51:713-725.
Maresova, P., E. Javanmardi, S. Barakovic, J. Barakovic Husic, S. Tomsone, O. Krejcar, and K. Kuca (2019). Consequences of chronic diseases and other limitations associated with old age - a scoping review. BMC Public Health 19:1431.
Matz, R., C. Schott, J. Stoclet, and R. Andriantsitohaina (2000). Age-related endothelial dysfunction with respect to nitric oxide, endothelium-derived hyperpolarizing factor and cyclooxygenase products. Physiol. Res. 49:1-18.
Moore, D.R., M.J. Robinson, J.L. Fry, J.E. Tang, E.I. Glover, S.B. Wilkinson, T. Prior, M.A. Tarnopolsky, and S.M. Phillips (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am. J. Clin. Nutr. 89:161-168.
Moore, D.R., T.A. Churchward-Venne, O. Witard, L. Breen, N.A. Burd, K.D. Tipton, and S.M. Phillips (2015). Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A Biol. Sci. Med. Sci. 70:57-62.
Murphy, C.H., and C. McGlory (2021). Fish oil for healthy aging: Potential application to master athletes. SSE #221.
Oikawa, S.Y., T.D. Brisbois, L.J.C. van Loon, and I Rollo. Eat like an athlete: insights of sports nutrition science to support active aging in healthy older adults. GeroSci. 43:2485-2495, 2021.
Phillips, B., J. Williams, P. Atherton, K. Smith, W. Hildebrandt, D. Rankin, P. Greenhaff, I. Macdonald, and M.J. Rennie (2012). Resistance exercise training improves age-related declines in leg vascular conductance and rejuvenates acute leg blood flow responses to feeding and exercise. J. Appl. Physiol. 112:347-353.
Phillips, S.M., J.E. Tang, and D.R. Moore (2009). The role of milk-and soy-based protein in support of muscle protein synthesis and muscle protein accretion in young and elderly persons. J. Am. Coll. Nutr. 28:343-354.
Philpott, J.D., O.C. Witard, and S.D.R. Galloway (2019). Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 27:219-237.
Reid, K.F., E. Pasha, G. Doros, D.J. Clark, C. Patten, E.M. Phillips, W.R. Frontera, and R.A. Fielding (2014). Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur. J. Appl. Physiol. 114:29-39.
Rodacki, C.L., A.L. Rodacki, G. Pereira, K. Naliwaiko, I. Coelho, D. Pequito, and L.C. Fernandes (2012). Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 95:428-436.
Rollo, I., J.T. Gonzalez, C.J. Fuchs, L.J.C. van Loon, and C. Williams (2020). Primary, secondary, and tertiary effects of carbohydrate ingestion during exercise. Sports Med. 50:1863-1871.
Scarmeas, N., C.A. Anastasiou, and M. Yannakoulia (2018). Nutrition and prevention of cognitive impairment. Lancet Neurol. 17:1006-1015.
Sharda, M., P. Jain, A. Gupta, D. Nagar, and A. Soni. (2015). Correlation and comparison of various anthropometric measurements of body fat distribution and sagittal abdominal diameter as a screening tool for cardio metabolic risk factors and ischaemic heart disease in elderly population. J. Assoc. Physicians India 63:22-26.
Smith, G.I., S. Julliand, D.N. Reeds, D.R. Sinacore, S. Klein, and B. Mittendorfer (2015). Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 102:115-122.
Terjung, R.L., P. Clarkson, E.R. Eichner, P.L. Greenhaff, P.J. Hespel, R.G. Israel, W.J. Kraemer, R.A. Meyer, L.L. Spriet, M.A. Tarnopolsky, A.J.M. Wagenmakers, and M.H. Williams (2000). American College of Sports Medicine roundtable. The physiological and health effects of oral creatine supplementation. Med. Sci. Sports Exerc. 32706-717.
Thomas, D.T., K.A. Erdman, and L.M. Burke (2016). Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 116:501-528.
Trombetti, A., K.F. Reid, M. Hars, F.R. Herrmann, E. Pasha, E.M. Phillips, and R.A. Fielding (2016). Age-associated declines in muscle mass, strength, power, and physical performance: impact on fear of falling and quality of life. Osteoporos Int. 27:463-471.
Turner, C.E., W.D. Byblow, and N. Gant (2015). Creatine supplementation enhances corticomotor excitability and cognitive performance during oxygen deprivation. J. Neurosci. 35:1773-1780.
van Loon, L.J.C. (2017). Dietary protein to support active aging. SSE #160.
Wickham, K.A., and L.L. Spriet (2019). No longer beeting around the bush: a review of potential sex differences with dietary nitrate supplementation. Appl. Physiol. Nutr. Metab. 44:915-924.
Wickham, K.A., D.G. McCarthy, J.M. Pereira, D.T. Cervone, L.B. Verdijk, L.J.C. van Loon, G.A. Power, and L.L. Spriet (2019). No effect of beetroot juice supplementation on exercise economy and performance in recreationally active females despite increased torque production. Physiol. Rep. 7:e13982.
Wightman, E.L., C.F. Haskell-Ramsay, K.G. Thompson, J.R. Blackwell, P.G. Winyard, J. Forster, A.M. Jones, and D.O. Kennedy (2015). Dietary nitrate modulates cerebral blood flow parameters and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Physiol. Behav. 149:149-158.
Williams, C., and I. Rollo (2015). Carbohydrate nutrition and team sport performance. Sports Med. 45(Suppl 1):S13-S22.
World population ageing 2019. (2020). 36 p.
Yang, Y., L. Breen, N.A. Burd, A.J. Hector, T.A. Churchward-Venne, A.R. Josse, M.A. Tarnopolsky, and S.M. Phillips (2012). Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 108:1780-1788.
Zampieri, S., L. Pietrangelo, S. Loefler, H. Fruhmann, M. Vogelauer, S. Burggraf, A. Pond, M. Grim-Stieger, J. Cvecka, M. Sedliak, V. Tirpakova, W. Mayr, N. Sarabon, K. Rossini, L. Barberi, M. De Rossi, V. Romanello, S. Boncompagni, A. Musaro, M. Sandri, F. Protasi, U. Carraro, and H. Kern (2015). Lifelong physical exercise delays age-associated skeletal muscle decline. J. Gerontol. A Biol. Sci. Med. Sci. 70:163-173.