KEY POINTS
- Athletes are exposed to many stressors that can increase injury and illness risk and cause excessive fatigue. These include physical workload, sleep loss, travel, and psychological stress.
- Two synergistic approaches to blood biomarker analysis in sport, profiling and monitoring, offer an opportunity to gain insight into an athlete’s nutritional and physiological status.These approaches may help to avoid overtraining, injury and illness when combined with other contextual information.
- Common problems that can be identified via blood test data include: poor vitamin D and iron status, low energy availability, persistent inflammation, persistent oxidative stress, and decreased hormonal drive.
- Blood test data are easily compromised by poor pre-analytic approaches, for example, drawing blood following exercise or a meal. In order for data to be valid and reliable, a range of pre-analytic considerations must be adhered to.
- Clinical diagnostic normative ranges have limited application in sport. Sport specific and athlete adaptive ranges, however, enable the identification of meaningful within-athlete changes that can inform nutrition and recovery strategies.
INTRODUCTION
The applied sport science and medicine team is tasked with protecting or enhancing an athlete’s health and resilience to injury or illness, while attempting to maximize the performance gains arising from physical conditioning. Some of the key variables that can be adjusted or influenced are the physical workload, diet and recovery strategies including sleep, nutrition, compression and cryotherapy etc. Subjective tools for monitoring athletes in order to inform how these variables should be adjusted can be convenient and cost effective, but can be compromised by inaccuracy, falsification or poor adherence over time. By contrast, blood biomarkers offer an objective approach to prioritizing the efforts of practitioners. However, these data can also be compromised by poor pre-analytic approaches, infrequent sampling and an inappropriate selection of biomarkers.
The evidence base supporting the use of blood biomarker analysis in sport has accumulated over the past 30 years or more (Pedlar et al., 2019). This Sports Science Exchange article describes some of the established and emerging biomarkers of most interest to sport science and medicine practitioners. Guidelines on how to collect the best possible quality data are provided, together with guidance on appropriate statistical techniques to assess longitudinal changes in biomarkers in athletes.
WHAT TO MEASURE?
Biomarker data collection and analysis provides grounds for interdisciplinary communication and collaboration between sports medicine and sport science staff. For example, vitamin D status is of significant interest to both medical and nutritional staff in the care of an athlete with a history of bone injury. Similarly, energy status markers are of interest to physiology, nutritional and medical staff in the case of a fatigued endurance athlete.
Biomarkers of Nutritional Status
Broadly, a host of nutrition-related biomarkers can be assessed within the blood with certain limitations and caveats surrounding each nutrient. Measuring nutrients in blood components (red cells, white cells, serum) can reduce time-consuming dietary recall and analysis approaches. Larson-Meyer et al. (2018) provided a comprehensive guide to assessing each nutrient via the analysis of biomarkers; in the subsequent section we review some of the selected examples of interest.
Iron
Adequate iron intake and storage underpins erythropoiesis or the production of new red blood cells (RBC) and the maintenance or increase in total hemoglobin mass with endurance training, particularly at altitude. If left unchecked, low iron stores can lead to iron-deficient anaemia with profound effects on endurance performance. Females are particularly at risk of iron deficiency due to menstrual blood losses (Pedlar et al., 2018). Iron status is typically assessed via the measurement of serum ferritin, which is broadly the best marker of iron storage; however, it is possible that ferritin can be relatively low and the athlete can continue to adapt (Pedlar et al., 2013). Hemoglobin concentration is also a key variable which can appear low due to the hemodilution (plasma volume expansion) that accompanies training. Therefore, total hemoglobin mass measurements are recommended, but when not available, RBC morphology markers, to identify microcytic (low volume) and/or hypochromic (low hemoglobin) cells, are recommended to identify a functional iron deficiency (Archer & Brugnara, 2015; Burden et al., 2015). Recently hepcidin has emerged as a key marker of iron metabolism, providing an indicator of iron absorption. Supplementing with iron results in an acute increase in hepcidin, orchestrating a reduced iron absorption. It is feasible that it is not worth supplementing with iron with a raised hepcidin present, since absorption will be compromised (Stoffel et al., 2020). However, it is important to note that exercise also transiently increases hepcidin, particularly when exercise inflammation is present (Peeling et al., 2014). Further work is needed in this area and hepcidin measurement is not yet widely available in clinical laboratories.
Vitamin D
Vitamin D has emerged as an important biomarker for athletes (Owens et al., 2018). Low vitamin D status, particularly common in northern latitudes where sunlight exposure is low, has been linked to poor immune function and compromised bone health and muscle repair. Recent work has highlighted the limitations of the established vitamin D assay (25-OHD), in the context of bone health, since black individuals have been observed to have comparable free bioavailable vitamin D concentrations despite significantly lower total 25-OHD levels (Allison et al., 2018). Therefore, where the assay is available, it is the vitamin D bioavailable form (vitamin D-binding protein) that should be measured. Vitamin D deficiency is clearly associated with compromised immunity and increased upper respiratory tract infections (He et al., 2016), and is easily corrected via nutritional approaches.
Fatty Acids
The assessment of the fatty acids, docosahexaenoic acid (DHA, C22:6) and eicosapentaenoic acid (EPA, C20:5), that are incorporated into RBC membranes has become common in athletes, despite a relatively sparse evidence base. The omega-3 index (OM3I), a validated, reliable and reproducible biomarker for the assessment of omega-3 status, represents the percentage of the long chain omega fatty acids EPA and DHA as a proportion (%) of the total RBC fatty acids (Harris, 2010). Red cell fatty acids reflect dietary intake over the previous month, and as such these can provide valuable insights into the quality of an athlete’s diet. Early work identified the potential for fatty acids to modify inflammation (Calder, 2017); however, several systems and/or functions may be influenced by fatty acid status including mood and cognition (Fontani et al., 2005), muscle recovery (Black et al., 2018), lung function (Mickleborough et al., 2003), concussion (Oliver et al., 2016) and cardiovascular function (Hingley et al., 2017). The research evidence as it pertains to athletes is summarized in a recent systematic review (Lewis et al., 2020a).
Energy Availability
Maintaining energy availability is critical for avoiding the many possible negative outcomes associated with energy deficiency, as recently proposed in the Relative Energy Deficiency in Sport (RED-S) framework (Mountjoy et al., 2018). There are several peptide hormones and cytokines that serve as indicators of energy availability and have been associated with prolonged endurance training including leptin, ghrelin, Interleukin (IL)-6 and tumour necrosis factor alpha (Jurimae et al., 2011). Reduced total triiodothyronine has more recently been clearly related to energy status and training adaptation in female swimmers (Vanheest et al., 2014), and demonstrably responds to reduced energy intake in males (Friedl et al., 2000). Testosterone also declines with energy deficiency (Friedl et al., 2000) and is rapidly restored with increased dietary carbohydrate ingestion (Lane et al., 2010).
Monitoring biomarkers to inform training load
Understanding when to increase training load and when to reduce training load is a perennial challenge for coaches and athletes. Using point of care (POC) blood tests offers an opportunity for rapid results that can be immediately accessible to the sport scientist to assess recovery, if consistently collected. Biomarkers of oxidative stress (for example lipid and protein hydroperoxides, isoprostanes, protein carbonyls), inflammation (e.g., IL-6, C-reactive protein), muscle damage (e.g., creatine kinase) and hormonal drive (e.g., testosterone, cortisol) can inform the decision to increase or decrease training load. Unfortunately, only a few of these are available as POC tests.
Hormesis is a term used in toxicology which refers to a dose response curve where a low dose provides inadequate stimulation, and a high dose has an inhibitory or toxic effect. Consider a hormetic set point for the athlete in training, that is influenced by the sum of all stressors (broadly increasing hormesis) including metabolic, environmental, mechanical, psychological, immunological stressors and the sum of all practices which support recovery (broadly reducing the hormetic set point) including nutrition, sleep, compression, cryotherapy. If the aforementioned biomarkers are measured frequently, they can provide an indicator of the hormetic set point. Clearly this is a simplified framework and more research is needed to define the influence of each of these variables. However, hormetic models have been described by several authors who have asserted that drifting too far above this hormetic set point increases the risk of overtraining, injury and illness, and decreases the capacity to adapt (Peake et al., 2015; Pingitore et al., 2015; Slattery et al., 2015). Our recent work demonstrated that with evidence of higher oxidative stress, the risk of illness and injury increases proportionally (Lewis et al., 2020b). Several other studies have demonstrated an interaction between training load and these biomarkers, including: 1) continuously increasing training load with insufficient recovery in cyclists, resulting in elevated oxidative stress and a performance plateau (Knez et al., 2014); 2) increasing training load in female swimmers with low energy availability resulting in reduced bioenergetic hormones (total triiodothyronine, insulin-like growth factor) and maladaptation (Vanheest et al., 2014); and 3) various biomarkers (oxidative stress, immune function and nutritional status) fluctuating throughout a season in professional rugby players, with greatest disturbances observed during intensified training (Finaud et al., 2006).
Adaptation to aerobic exercise training can be quantified by measuring the blood lactate response to controlled exercise bouts, i.e., a lower blood lactate response at a similar exercise intensity. Furthermore, reactive oxygen and nitrogen species (RONS) are becoming well established as important signalling molecules for training adaptation (Margaritelis et al., 2018). Measuring biomarkers of oxidative stress in response to exercise can offer insight into an athlete’s adaptive potential. A recent study stratified a large group (n=100) into low, moderate and high exercise-induced oxidative stress. Greater adaptations to a 6 week training programme were observed in the moderate and high groups in both aerobic and anaerobic exercise variables, indicating that at least a transient alteration in redox homeostasis, in this case described as oxidative stress, is required to stimulate adaptation (Margaritelis et al., 2018).
Risk of injury and illness
In a study by Lewis et al. (2020b), injury and illness were associated with a higher oxidative stress index in Olympic oarsmen. More specifically, the total antioxidant biomarker decreased with illness and a hydroperoxide biomarker increased with injury. Using a Cox proportional hazard model, a 0.5 mmol·L-1 increase in the total anti-oxidant biomarker exerted a ˜30% protective effect on illness (Lewis et al., 2020b). It is important to note that although these assays are well suited to the applied sport science setting, with high precision and POC convenience (Lewis et al., 2016), the over-simplification of establishing an index of oxidative stress from these types of assays has been cautioned against (Cobley et al., 2017) and therefore the data should be interpreted with similar caution (Lewis et al., 2016).
In a sample of elite male and female distance runners, low T3 and low testosterone were associated with an increased risk of injury (Heikura et al., 2018). In particular, male athletes with testosterone values in the lower quartile of the sample, had a 4.5-fold higher stress fracture rate (Heikura et al., 2018). Interestingly, these athletes were within the normal clinical range for testosterone, demonstrating the poor utility of population based clinical reference ranges (see ANALYZING BIOMARKER DATA section below). These biomarkers provide objective data to inform recovery strategies, for example, improving the periodization of carbohydrate intake to address poor energy status.
Whilst associations between injury or illness and biomarkers of energy status, sex hormones and oxidative stress are consistently reported, further work is needed to establish the predictive power of biomarker monitoring to reduce lost training days. However, this in itself is problematic due to the infrequent nature of injury and the many confounding variables in high-performance environments.
HOW TO SAMPLE BLOOD
Pre-analytic considerations
It is of paramount importance to collect blood using a consistent methodology, adhering to pre-analytic rules, if data are to be useful for detecting changes across time. The distribution of the constituents of blood are dramatically altered with exercise. If the exercise is prolonged, unaccustomed or excessive, there can be evidence of this in the blood for several days afterwards (Hill et al., 2014). Posture also results in marked changes in hematocrit, e.g., prone vs. seated vs. standing (Lippi et al., 2015). An overview of all pre-analytic considerations together with a set of eight simple recommendations to improve the quality of blood test data is presented in Figure 1.
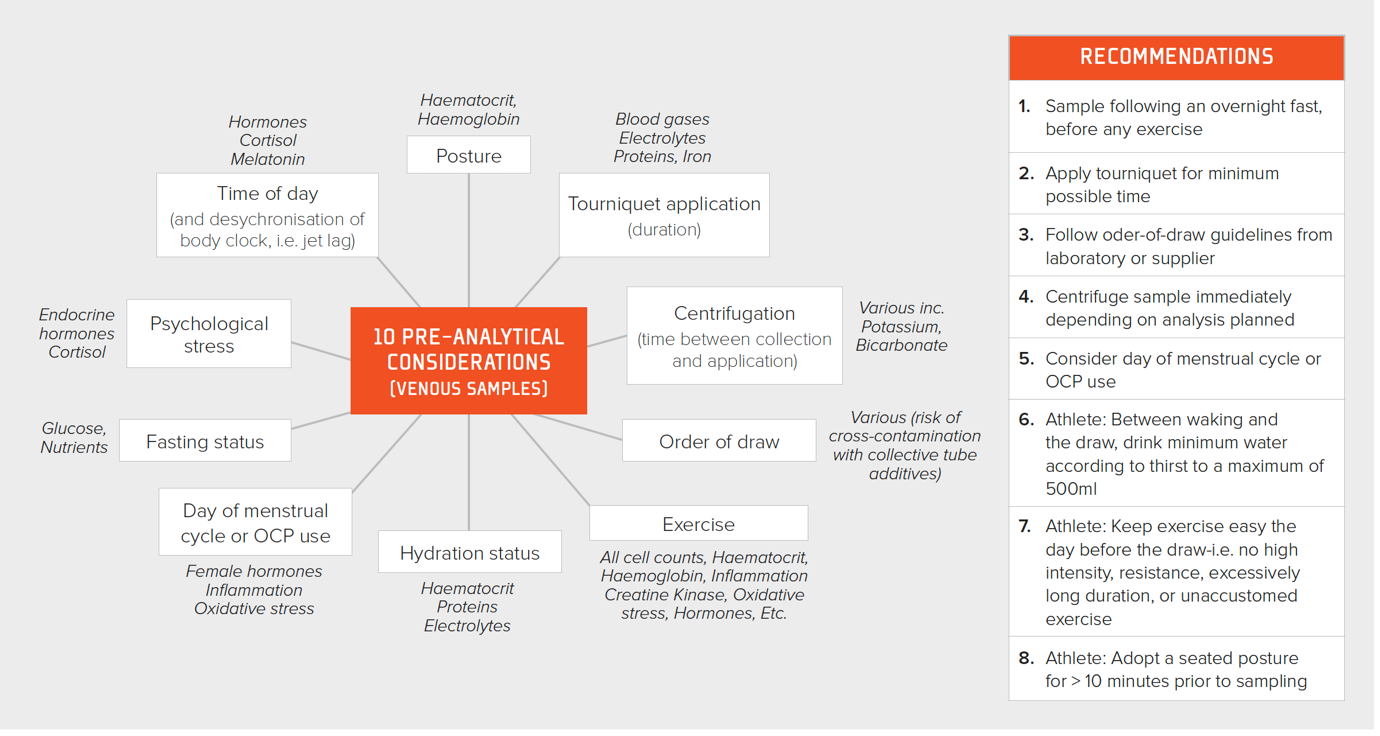
Figure 1. Pre-analytic considerations for improving the quality of blood sampling together with recommendations for the practitioner. OCP = oral contraceptive pill.
It is advisable to remove as little blood as possible from the athlete. To give this context, it is well known that female athletes are at a higher risk of iron deficiency due to menstrual blood losses (light flow = < 36.5 mL per cycle; heavy flow ~72.5 mL per cycle), which can be similar to the amount of blood lost with a comprehensive blood draw, depending on the efficiency of the lab.
Analyzing Biomarker data
Normal Ranges
The identification of departures from population-based reference ranges is a primary objective in individual-level monitoring of biomarkers in medicine and in elite sports. The time-evolution of the data is often ignored with individual data points compared against fixed population-derived reference ranges (e.g., normal ranges) based on a healthy cross-sectional sample of the general population. In reality, typical biomarker levels in elite athletes are different compared to the “general” population at large, and normative ranges should therefore be derived from a population of elite athletes where possible. Accumulating sport-specific or even position-specific data can be useful to establish more useful cutoffs to aid interpretation of biomarker data in the applied sport setting. Precious few examples of this exist in the literature, e.g., creatine kinase (Mougios, 2007) and iron status (Mettler and Zimmermann, 2010), although the Australian Institute of Sport (1999) published normative ranges for athletes some 20 years ago. Furthermore, athletes are often extreme phenotypic outliers with large intra-individual differences, and therefore even athlete based normative data can be of little practical use and individual ranges are likely more appropriate.
Longitudinal analysis
Repeated measurements on the same athletes offer a major statistical advantage, and analyses should capitalize on the frequency of data derived from longitudinal monitoring, with information on within athlete variability informing interpretation. Bayesian approaches are a natural choice where prior individual measurements are taken into account to construct personalized reference ranges from monitoring data, that adapt to an individual’s variability over time (Figure 2).
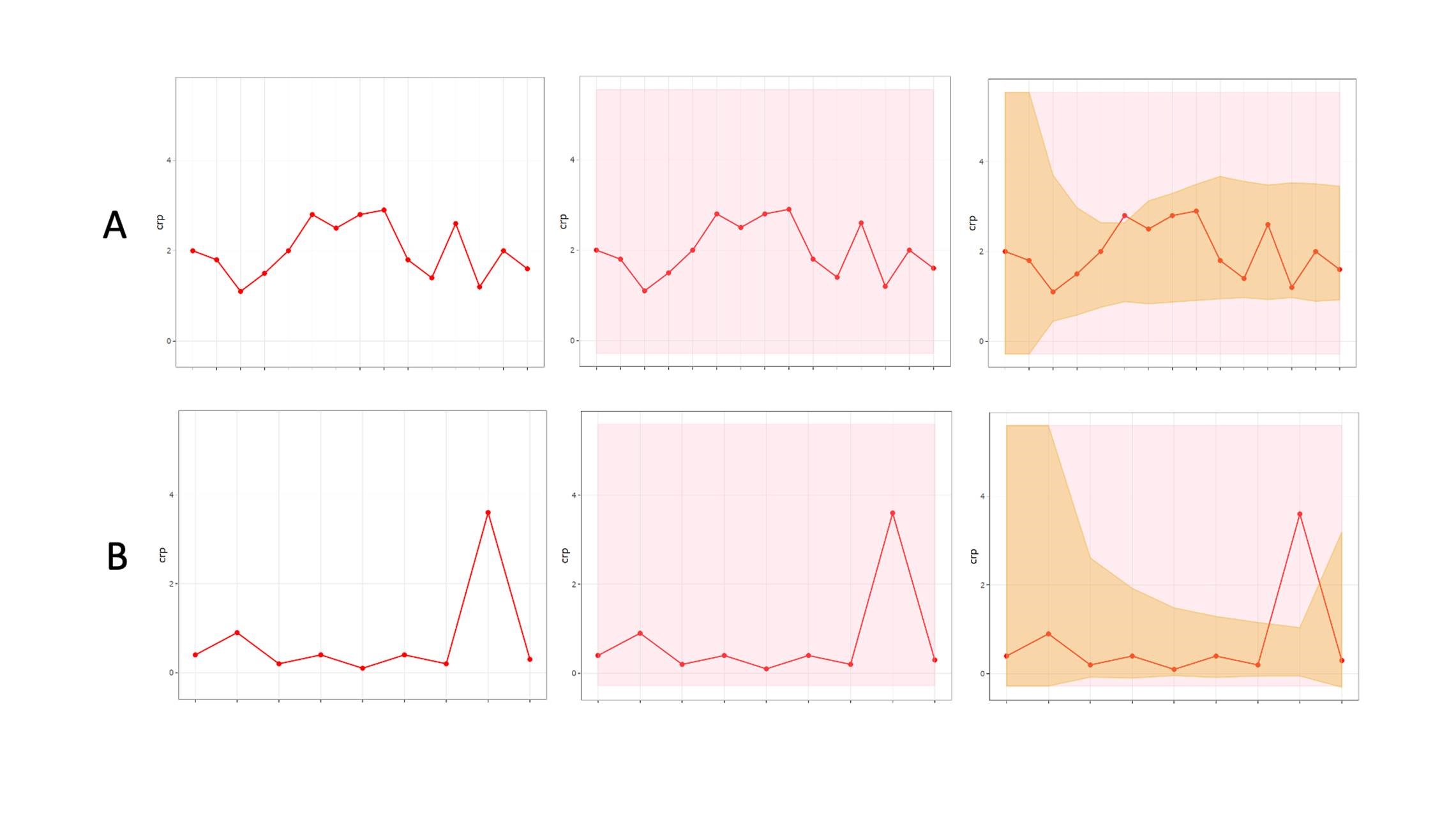
Figure 2: An individualized adaptive range (brown shading) providing greater diagnostic accuracy within a population based clinical reference range (pink shading), for two biomarkers “A” and “B.”
Bayesian approaches for athlete monitoring have been employed to establish individual normative ranges, as is the case with the “Athlete Biological Passport” (Sottas et al., 2010). Some examples of this approach being deployed by sport scientists are appearing in the literature. Hecksteden and colleagues (2017) created individualized ranges for biomarkers of muscle recovery (creatine kinase and urea) in both “recovered” (pre-match) and “non-recovered” (post-match) states, providing the potential for superior diagnostic accuracy (i.e., poor recovery) within an individual athlete. In addition, through the application of critical difference values, meaningful changes can be identified within an individual since biological variation and measurement error are accounted for (Lewis et al., 2016).
PRACTICAL APPLICATIONS
- Select appropriate biomarkers for athlete profiling, including nutritional assessment. Since these comprehensive biomarker panels require a venous blood draw, they should only occur infrequently, e.g., 4 times per year.
- Select appropriate biomarkers for monitoring, point of care tests where possible. These can provide an indication of an athlete’s recovery status on a more frequent basis, e.g., once per week.
- Pre-analytic considerations for blood sampling are essential to ensure that good quality, reliable data are collected and increasing the chances of identifying a physiological change in an athlete.
- Blood biomarkers only provide an indication of physiological status at the time of the test. They should be combined with other data including subjective, physical and metabolic data to truly inform practice.
SUMMARY
Assessing biomarkers in athletes provides a collaborative space for sports nutrition, physiology and medicine staff to understand the recovery status of the athlete and prioritize strategic interventions, with the goal of reducing days lost to injury and illness and maximizing training outcomes. Clear evidence exists of the negative outcomes associated with poor vitamin D status, iron status and energy availability. Appropriate (selection, collection technique, measurement frequency, statistical interpretation) biomarker analysis can provide objective insight into these issues.
REFERENCES
Allison, R.J., A. Farooq, A. Cherif, B. Hamilton, G.L. Close, and M. G. Wilson (2018). Why don't serum vitamin D concentrations associate with BMD by DXA? A case of being 'bound' to the wrong assay? Implications for vitamin D screening. Br. J. Sports Med. 52:522-526.
Archer, N.M., and C. Brugnara (2015). Diagnosis of iron-deficient states. Crit. Rev. Clin. Lab. Sci. 52:256-272.
Australian Institute of Sport, AIS. (1999). Sports Haematology Laboratory, sports haematology and biochemistry handbook (Australian Sports Commission: Canberra, Australia).
Black, K.E., O.C. Witard, D. Baker, P. Healey, V. Lewis, F. Tavares, S. Christensen, T. Pease, and B. Smith (2018). Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. Eur. J. Sport Sci,.18:1357-1367.
Burden, R.J., N. Pollock, G.P. Whyte, T. Richards, B. Moore, M. Busbridge, S.K. Srai, J. Otto, and C.R. Pedlar (2015). Effect of intravenous iron on aerobic capacity and iron metabolism in elite athletes. Med. Sci. Sports Exerc. 47:1399-1407.
Calder, P.C. (2017). Omega-3 fatty acids and inflammatory processes: from molecules to man, Biochem. Soc. Trans. 45:1105-1115.
Cobley, J.N., G.L. Close, D.M. Bailey, and G.W. Davison (2017). Exercise redox biochemistry: Conceptual, methodological and technical recommendations. Redox Biol. 12:540-548.
Finaud, J., V. Scislowski, G. Lac, D. Durand, H. Vidalin, A. Robert, and E. Filaire (2006). Antioxidant status and oxidative stress in professional rugby players: evolution throughout a season. Int. J. Sports Med. 27:87-93.
Fontani, G., F. Corradeschi, A. Felici, F. Alfatti, S. Migliorini, and L. Lodi (2005). Cognitive and physiological effects of Omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur. J. Clin. Invest. 35:691-699.
Friedl, K.E., R.J. Moore, R.W. Hoyt, L.J. Marchitelli, L.E. Martinez-Lopez, and E.W. Askew (2000). Endocrine markers of semistarvation in healthy lean men in a multistressor environment. J. Appl. Physiol. 88:1820-1830.
Harris, W.S. (2010). The omega-3 index: clinical utility for therapeutic intervention. Curr. Cardiol. Rep. 12:503-508.
He, C.S., X.H. Aw Yong, N.P. Walsh, and M. Gleeson (2016). Is there an optimal vitamin D status for immunity in athletes and military personnel? Exerc. Immunol. Rev. 22:42-64.
Hecksteden, A., W. Pitsch, R. Julian, M. Pfeiffer, M. Kellmann, A. Ferrauti, and T. Meyer (2017). A new method to individualize monitoring of muscle recovery in athletes. Int. J. Sports Physiol. Perform. 12:1137-1142.
Heikura, I.A., A.L.T. Uusitalo, T. Stellingwerff, D. Bergland, A.A. Mero, and L.M. Burke (2018). Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int. J. Sport Nutr. Exerc. Metab. 28:403-411.
Hill, J.A., G. Howatson, K.A. van Someren, I. Walshe, and C.R. Pedlar (2014). Influence of compression garments on recovery after marathon running. J. Strength Cond. Res. 28:2228-2235.
Hingley, L., M.J. Macartney, M.A. Brown, P.L. McLennan, and G.E. Peoples (2017). DHA-rich fish oil increases the omega-3 index and lowers the oxygen cost of physiologically stressful cycling in trained individuals. Int. J. Sport Nutr. Exerc. Metab. 27:335-343.
Jurimae, J., J. Maestu, T. Jurimae, B. Mangus, and S.P. von Duvillard (2011). Peripheral signals of energy homeostasis as possible markers of training stress in athletes: a review. Metabolism 60:335-350.
Knez, W.L., D.G. Jenkins, and J.S. Coombes (2014). The effect of an increased training volume on oxidative stress. Int. J. Sports Med. 35:8-13.
Lane, A.R., J.W. Duke, and A.C. Hackney (2010). Influence of dietary carbohydrate intake on the free testosterone: cortisol ratio responses to short-term intensive exercise training. Eur. J. Appl. Physiol. 108:1125-1131.
Larson-Meyer, D.E., K. Woolf, and L.M. Burke (2018). 'Assessment of nutrient status in athletes and the need for supplementation. Int. J. Sport Nutr. Exerc. Metab. 28:139-158.
Lewis, N.A., J. Newell, R. Burden, G. Howatson, and C.R. Pedlar (2016). Critical difference and biological variation in biomarkers of oxidative stress and nutritional status in athletes. PLoS One 11:e0149927.
Lewis, N.A., D. Daniels, P.C. Calder, L. Castell, and C.R. Pedlar (2020a). Are there benefits for the use of fish oil (omega-3) supplements in athletes? A systematic review. Adv. Nutr. In Press.
Lewis, N.A., A.J. Simpkin, S. Moseley, G. Turner, M. Homer, A. Redgrave, C.R. Pedlar, and R. Burden (2020b). Increased oxidative stress in injured and ill elite international olympic rowers. Int. J. Sports Physiol. Perform. Epub ahead of print.
Lippi, G., G.L. Salvagno, G. Lima-Oliveira, G. Brocco, E. Danese, and G.C. Guidi (2015). Postural change during venous blood collection is a major source of bias in clinical chemistry testing. Clin. Chim. Acta 440:164-168.
Margaritelis, N.V., A.A. Theodorou, V. Paschalis, A.S. Veskoukis, K. Dipla, A. Zafeiridis, G. Panayiotou, I.S. Vrabas, A. Kyparos, and M.G. Nikolaidis (2018). Adaptations to endurance training depend on exercise-induced oxidative stress: exploiting redox interindividual variability. Acta Physiol. 222:12898.
Mettler, S., and M.B. Zimmermann (2010). Iron excess in recreational marathon runners. Eur. J. Clin. Nutr. 64:490-494.
Mickleborough, T.D., R.L. Murray, A.A. Ionescu, and M.R. Lindley (2003). Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am. J. Respir. Crit. Care Med. 168:1181-1189.
Mougios, V. (2007). Reference intervals for serum creatine kinase in athletes. Br. J. Sports Med. 41 674-678.
Mountjoy, M., J.K. Sundgot-Borgen, L.M. Burke, K.E. Ackerman, C. Blauwet, N. Constantini, C. Lebrun, B. Lundy, A.K. Melin, N.L. Meyer, R.T. Sherman, A.S. Tenforde, M. Klungland Torstveit, and R. Budgett (2018). IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 52:687-697.
Oliver, J.M., M.T. Jones, K.M. Kirk, D.A. Gable, J.T. Repshas, T.A. Johnson, U. Andréasson, N. Norgren, K. Blennow, and H. Zetterberg (2016). Effect of docosahexaenoic acid on a biomarker of head trauma in American football. Med. Sci. Sports Exerc. 48:974-982.
Owens, D.J., R. Allison, and G.L. Close (2018). Vitamin D and the athlete: Current perspectives and new challenges. Sports Med. 48:3-16.
Peake, J.M., J.F. Markworth, K. Nosaka, T. Raastad, G.D. Wadley, and V.G. Coffey (2015). Modulating exercise-induced hormesis: Does less equal more? J. Appl. Physiol. 119:172-189.
Pedlar, C.R., G.P. Whyte, R. Burden, B. Moore, G. Horgan, and N. Pollock (2013). A case study of an iron-deficient female Olympic 1500-m runner. Int. J. Sports Physiol. Perform. 8:695-698.
Pedlar, C.R., C. Brugnara, G. Bruinvels, and R. Burden (2018). Iron balance and iron supplementation for the female athlete: A practical approach. Eur. J. Sport Sci. 18:295-305.
Pedlar, C.R., J. Newell, and N.A. Lewis (2019). Blood biomarker profiling and monitoring for high performance physiology and nutrition: current perspectives, limitations and recommendations. Sports Med. 49(Suppl 2):185-198.
Peeling, P., M. Sim, C.E. Badenhorst, B. Dawson, A.D. Govus, C.R. Abbiss, D.W. Swinkels, and D.
Trinder (2014). Iron status and the acute post-exercise hepcidin response in athletes. PLoS
One 9:e93002.
Pingitore, A., G.P. Lima, F. Mastorci, A. Quinones, G. Iervasi, and C. Vassalle (2015). Exercise and oxidative stress: potential effects of antioxidant dietary strategies in sports. Nutrition 31:916-922.
Slattery, K., D. Bentley, and A.J. Coutts (2015). The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 45:453-471.
Sottas, P.E., N. Robinson, and M. Saugy (2010). The athlete's biological passport and indirect markers of blood doping. Handb. Exp. Pharmacol. pp. 305-326.
Stoffel, N.U., C. Zeder, GM. Brittenham, D. Moretti, and M. B. Zimmermann (2020). Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women, Haematologica. 105:1232-1239.
Vanheest, J.L., C.D. Rodgers, C.E. Mahoney, and M.J. De Souza (2014). Ovarian suppression impairs sport performance in junior elite female swimmers. Med. Sci. Sports Exerc. 46:156-166.