KEY POINTS
- Dietary protein provides amino acid building blocks to repair, remodel, and build (synthesize) muscle and body proteins. Amino acids may also represent a minor source of energy during exercise and therefore must be replenished through the diet.
- There is little evidence that master athletes metabolize dietary protein differently than younger athletes, allowing for easier translation of our current understanding of protein requirements that has been established almost exclusively in young adults.
- A single meal protein dose of 0.3-0.4 g/kg will maximize muscle protein synthesis at rest and during post-exercise recovery, although endurance athletes should consume up to 0.5 g/kg immediately after exercise to replenish any amino acid oxidative losses (use for energy).
- Consuming four balanced, protein-containing meals will be the most efficient protein intake pattern to support rates of muscle protein synthesis. These meals should focus on nutrient-dense foods, although supplemental forms of leucine-enriched proteins may be a convenient means to enhance muscle remodeling on the go.
- Education of master athletes on these protein recommendations may represent a practical and effective means to support their training recovery and performance goals.
Introduction
Dietary protein is essential for an athlete’s recovery and adaptation as it provides the requisite amino acid building blocks to repair and remodel old and/or damaged proteins, especially within contracting skeletal muscle. Amino acids may also be used as a minor source of fuel during exercise that requires high mitochondrial flux (repeated sprint and steady state endurance exercise) and therefore must be consumed in the diet to replenish these exercise-induced losses of the essential amino acids (branched chain amino acids).
Athlete performance is intimately linked to the quantity and quality of skeletal muscle. Muscle mass typically peaks for an athlete during their twenties or thirties and is relatively stable through their forties and perhaps into their fifties, provided training is maintained. During this middle aged period in which athletes may technically still qualify as master athletes (i.e., >35 y), available evidence suggests their protein requirements are indistinguishable from athletes half their age (Meredith et al., 1989). However, regardless of training, muscle mass and exercise performance begins to wane, with losses generally accelerating into the seventh decade. The typical age-related loss of muscle mass is multifactorial but is generally rooted in an inability of skeletal muscle to respond to and utilize dietary amino acids to build new muscle proteins, which is referred to as anabolic resistance. Thus, in comparison with their younger counterparts, older untrained adults require up to 60% more protein in a single meal to maximally stimulate muscle protein synthesis at rest (Moore et al., 2015) or after a typical bout of resistance exercise (Witard et al., 2014; Yang et al., 2012). This has led to the suggestion that older adults require a greater amount of protein per meal (and thus daily intake) to overcome this anabolic resistance to help maintain muscle mass (Figure 1). However, the high training volumes of most master athletes sets them apart from their sedentary peers and calls into question whether, in light of the lack of research on the topic, their protein requirements are indeed elevated above their younger trained peers, as will be discussed in this Sports Science Exchange article.
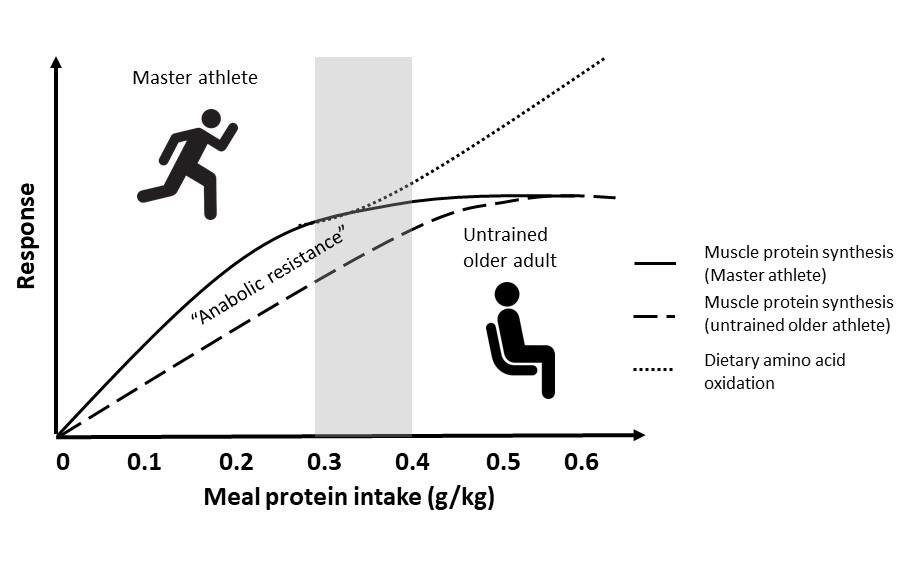
Little evidence for anabolic resistance in Master athletes
In general, anabolic resistance is intimately linked to the activity status of muscle. Extreme forms of inactivity (e.g., bedrest, immobilization) result in a severe blunting of the muscle protein synthetic response to exogenous amino acids independent of age (Oikawa et al., 2019; Phillips et al., 2009), and mild forms of disuse (e.g., reduced daily step count) may underpin or exacerbate the anabolic resistance of aging (Breen et al., 2013; Moore, 2014). In contrast, exercise such as brief walking (Timmerman et al., 2012) or light load resistance exercise (Devries et al., 2015) can increase the anabolic sensitivity of muscle protein synthesis to dietary amino acids in older untrained adults. These observations alone ostensibly argue against any deleterious effects of aging per se on the response to and requirement for dietary protein in highly active master athletes. However, deconstructing the common causes of age-related anabolic resistance by first principles requires one to assess the mouth-to-muscle journey of dietary amino acids to determine whether any limitations exist in master athletes that could predispose them to differing protein requirements compared to their untrained peers or, more importantly, to younger athletes. This latter group forms the basis of sports science research into the acute and chronic requirements for this important macronutrient.
The first step in assimilating dietary amino acids into new muscle and body proteins is the ability to effectively digest and absorb these nutrients. Currently, there is little evidence that the slight attenuation in the appearance of dietary amino acids into circulation after protein ingestion that has been observed in healthy older adults represents a modifiable factor for age-related anabolic resistance (Gorissen et al., 2020). The gut has been suggested to be trainable in its ability to absorb nutrients such as carbohydrate (Jeukendrup, 2017), which may also extend to amino acids given there is no deleterious effect of prior endurance exercise on the ability to effectively absorb dietary protein during the post-exercise period in trained young athletes (Mazzulla et al., 2017). While there are no comparable data in older adults, there is little evidence that this would be compromised in master athletes either.
Once amino acids have been absorbed and enter the circulation, effective delivery to and uptake by the muscles is vital for ensuring optimal utilization of these dietary substrates for muscle repair and remodeling. Master athletes benefit from an extensive muscle capillary network that is similar to that of younger athletes (Coggan et al., 1990) which would efficiently dilate in response to insulin due to the high activity levels of this population (Fujita et al., 2007; Timmerman et al., 2012). Thus, the maintained vasodilatory capacity of master athletes would mitigate another predisposing factor in normal age-related anabolic resistance. Moreover, acute and chronic exercise is generally associated with increased amino acid transporter expression in older untrained adults (Dickinson et al., 2013), suggesting that the capacity for amino acid uptake would not be compromised in active master athletes. Much of the ability of amino acids to turn on muscle protein synthesis in older adults via the mechanistic target of rapamycin complex 1 (mTORC1) is also linked to the prior contraction of skeletal muscle (Timmerman et al., 2012), which would further argue against any age-related dysregulation in active, training master athletes. Therefore, previous suggestions of a greater protein requirement to maximize muscle protein remodeling at rest (Moore et al., 2015) or after resistance exercise (Doering et al., 2016b; Churchward-Venne et al., 2016) in older untrained adults are not relevant to master athletes maintaining an active training program. Thus, best practice for these athletes would be to follow the contemporary science and the requirements largely developed in younger athletes, with few caveats, as will be summarized below.
Protein requirements for resistance training
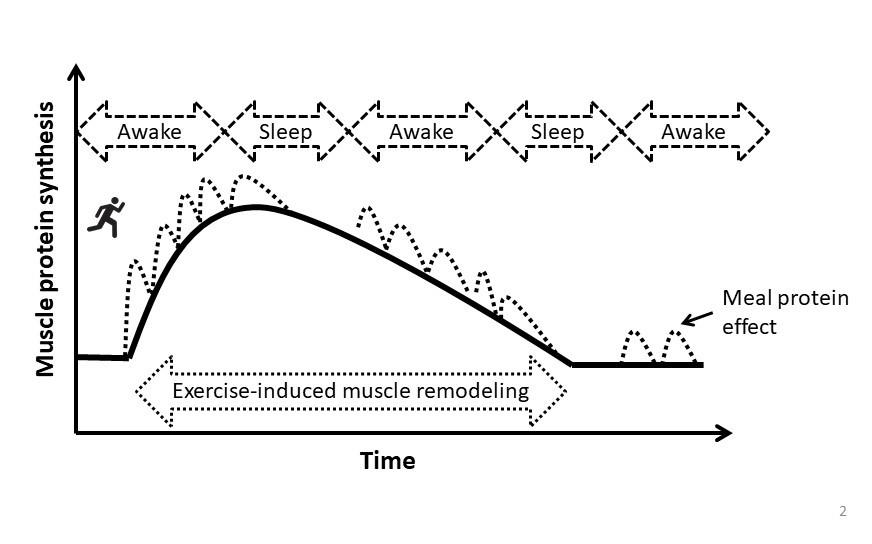
While the adequate consumption of carbohydrate is important to replenish muscle glycogen and support total daily energy needs, dietary protein is the most important macronutrient to support muscle anabolism after resistance exercise due to its ability to support maximal rates of muscle protein synthesis and attenuate the normal exercise-induced increase in muscle protein breakdown. Current evidence suggests that ~0.3 g protein/kg is a suitable target to maximize post-exercise muscle protein synthesis while minimizing its irreversible oxidation with excess consumption (Moore, 2019), although a common ~25% safety margin would put this at ~0.37 g protein/kg. This meal-protein target should be consumed 4 to 5 times per day, in equally spaced increments, to support muscle remodeling during the prolonged recovery period (Areta et al., 2013), although this balanced approach can also be applied to recovery days for the same benefit. Consuming the last meal of the day in close proximity to sleep will provide amino acid precursors to support muscle remodeling during the otherwise overnight fasted period (Snijders et al., 2019). This optimized meal protein intake approach (e.g., 5 meals at ~0.3 g/kg or 4 meals at ~0.37 g/kg) would provide a daily intake (~1.5 g/kg/d) that approximates the amount that has been suggested to maximize lean mass growth with training (~1.6 g/kg/d) (Morton et al., 2018). Thus, reverse engineering an optimal meal protein pattern could be to consume this daily target of ~1.6 g/kg/d over 4 to 5 equally spaced meals if this is simpler for dietary planning (Figure 2). However, the target should be nutrient-dense whole foods as the basis of the nutrition plan, with supplemental forms of protein that are leucine-enriched providing convenient nutrition for athletes on the go.
Protein requirements for endurance training
Similar meal protein distributions that are recommended for resistance-trained master athletes should also form the cornerstone for endurance athletes given their need to remodel skeletal muscle for optimal recovery. However, endurance exercise places additional nutritional stress on athletes for dietary protein given that there is an obligatory increase in amino acid oxidation with this exercise modality. While carbohydrate and fat represent the primary metabolic fuels during endurance exercise, amino acids provide ~5% of energy requirements that can increase to ~10% in low carbohydrate availability states (e.g., low muscle glycogen stores). Thus, protein ingestion not only supports muscle protein synthesis but also replaces these exercise-induced oxidative losses (Mazzulla et al., 2017), which collectively translate into a slightly greater post-exercise requirement of ~0.5 g/kg (Churchward-Venne et al., 2020). Thus, factoring this slightly greater post-exercise requirement into the balanced meal protein pattern would result in a daily target of ~1.8 g/kg/d. This intake has been shown to maximize whole body reconditioning during recovery (Kato et al., 2016) and sustain exercise performance during a period of increased training volume in young endurance athletes (Williamson et al., 2019). However, it is worth noting that master athletes consuming greater acute protein intakes (i.e., 3 × 0.6 g/kg vs. 3 × 0.3 g/kg over 6 h) during recovery between successive training bouts on the same day had marginally less strength loss (~5%) after this short-term recovery (Doering et al., 2017), which may have implications for athletes training multiple times per day.
Sex-based differences in protein requirements
The majority of research studying protein requirements in athletes of all ages has used male participants, which is a limitation for the field. Thus, it is often required to evaluate whether there are sex-based differences in exercise metabolism that could influence protein metabolism and requirements. Fluctuations in sex hormones in eumenorrheic premenopausal master female athletes can influence fat and amino acid metabolism, as estrogen has been shown to have a protein-sparing effect during endurance exercise (Hamadeh et al., 2005; Phillips et al., 1993). Therefore, protein requirements in premenopausal master female endurance athletes may be marginally (~15%) lower during the follicular phase when the estrogen/progesterone ratio is typically highest. However, athletes may experience hormonal fluctuations and inconsistent cycle lengths, highlighting that premenopausal female master athletes could adhere to current recommendations derived from research in males to ensure protein sufficiency regardless of their menstrual phase. Fortunately, resistance exercise elicits similar muscle protein synthetic responses between the sexes, suggesting that the post-exercise protein requirement for premenopausal female master athletes would be consistent with recommendations in males (Moore, 2019).
Menopause is characterized by an accelerated loss of muscle mass and the onset of a sexual dimorphism in rested muscle protein metabolism of untrained older adults (Smith et al., 2016), although the relevance of this to master athletes with high levels of physical activity is unclear. To date, there has been no research in post-menopausal master female athletes on which to base age- and sex-specific protein recommendations. Moreover, the acute protein requirement to enhance muscle protein synthesis after resistance exercise in older untrained females may be similar to (Oikawa et al., 2020) or slightly greater than (Devries et al., 2018) older untrained males (Yang et al., 2012), which does little to resolve the sex-specific recommendations for athletic populations. However, it is difficult to envision a scenario in which meal protein requirements for either endurance or resistance trained master female athletes are markedly different from their male counterparts. Therefore, it is recommended that post-menopausal master athletes also adhere to the recommended intakes for males.
Contemporary sports nutrition
Low Carbohydrate Availability Training
It is not uncommon (due to scheduling and/or planned periodized training) for endurance athletes to initiate training in a low carbohydrate availability state, such as before breakfast and/or after an overnight period of carbohydrate restriction, in order to alter fuel metabolism in favor of fatty acid oxidation and elicit enhanced mitochondrial biogenesis (Impey et al., 2018). However, commencing exercise with low glycogen availability can double the contribution of protein/amino acid oxidation as a source of fuel during endurance exercise with some estimates as high as ~10% of energy (Lemon et al., 1980). This has the predictable effect of increasing protein requirements by ~10% to 15% (depending on exercise intensity and duration) in young male athletes in order to replenish these oxidative losses (Gillen et al., 2019). However, these increased requirements, which may be satisfied by increasing each meal during the day by a marginal ~0.05 g/ kg of protein, are specific to endurance exercise, as low muscle glycogen has a relatively minor effect on anaerobic exercise that relies predominantly on the phosphocreatine system, such as high-intensity resistance exercise. Moreover, low muscle glycogen does not compromise the ability of dietary protein to support increased rates of muscle protein synthesis after resistance exercise in young adults (Camera et al., 2012). Therefore, master endurance athletes should be mindful of the daily and periodized carbohydrate intakes due to their potential impact on protein metabolism and requirements.
Time-Restricted Eating and Intermittent Fasting
Restricting daily calories to small windows throughout the day (e.g., 6-8 h) or alternating feeding and fasting days is gaining popularity as a means to reduce body fat by maintaining a lower 24-hour cumulative insulin response. When undertaken by endurance athletes, this periodized eating may blur the line with low carbohydrate availability training and/or recovery, which may be used as an attempt to accentuate metabolic stress and promote greater fat adaptation and/or mitochondrial biogenesis. However, this dietary approach is anathema to the balanced protein distribution that supports greater daily rates of muscle protein remodeling, as highlighted above. Time-restricted eating may contribute to the loss of both fat and lean mass in young endurance athletes over an 8-week protocol without any observable change in exercise performance (Brady et al., 2020). Given that master endurance athletes may not be protected from age-related muscle loss to the same degree as master strength athletes (Chambers et al., 2020; Coggan et al., 1990), this loss of lean mass in younger endurance athletes may give cause for concern in older athletes who may experiment with this dietary strategy. However, increases in fat free mass in young adults during resistance training do not seem to be attenuated with time-restricted eating (Stratton et al., 2020; Tinsley et al., 2017), which would be consistent with the anabolic nature of this exercise modality. Therefore, master endurance athletes may be better able to retain lean mass with time-restricted eating if they perform concurrent resistance training, although this remains to be determined. Nevertheless, prudent advice for master athletes, to avoid unintended muscle loss and support the highest rates of muscle remodeling, would be to prioritize (at least on most days) a balanced daily protein ingestion.
Protein type
It is commonly suggested that proteins that are rapidly digested and leucine enriched should be prioritized during the immediate post-exercise period given the ability of this essential amino acid to turn on muscle protein synthesis (Stokes et al., 2018). This typically lends to supplemental forms (e.g., drink, sports bar) of protein (e.g., whey) being prioritized, which may be convenient for some athletes’ lifestyles. However, there is growing support for an equivalent or potentially greater anabolic potential of whole foods compared with isolated protein sources (Burd et al., 2019). In addition, whole foods should represent the base of an athlete’s food plan given the nutritional value of nutrient-dense, minimally processed foods. Therefore, master athletes should prioritize a whole food approach to meet the meal protein target intakes but may consider leucine-enriched supplemental forms for convenience.
Scope for improvement in master athletes?
Master athletes generally report lower daily and acute post-exercise protein intakes than their younger counterparts (Di Girolamo et al., 2017; Doering et al., 2016a). Perhaps more critically, ~50% of master athletes responded that they “didn’t know” what their post-exercise protein targets should be, with only ~22% accurately reporting the previously suggested maximal dose of 20-25 g, although a distinct minority were consuming the revised target of ~0.5 g/kg (Doering et al., 2016a). Thus, knowledge translation and mobilization around the optimal protein targets may represent low-hanging fruit for master athletes to support their training, recovery, and performance goals.
Practical recommendations
- The most efficient means of consuming the daily protein requirement is to focus on meal protein intake, which should be at least 0.3 g/kg and at most 0.5 g/kg per meal.
- Meals should ideally be spaced by ~4 h and include four eating occasions, including one in proximity (< 2 h) to bedtime if it does not disrupt sleep (may need to trial).
- Nutrient- and protein-dense whole foods should be prioritized, with leucine-enriched supplemental protein sources consumed out of convenience (e.g., on the go, immediately post-exercise).
- This balanced daily protein ingestion will optimize muscle protein remodeling and lean body mass, but additional research is needed to clearly identify how these align with sports performance outcomes.
- Educating master athletes of these protein recommendations may represent a practical approach to help them optimize their training recovery and adaptation.
Conclusion
Master athletes represent a sizeable and growing population of athletes but with minimal direct research characterizing their protein needs. However, there is little evidence that their response to or requirements for dietary protein would differ from their younger, athletic counterparts given the ability of exercise to lower older adults’ biological age and maintain their muscles’ sensitivity to dietary amino acids. Focusing on a balanced distribution of moderate protein-containing, nutrient-dense meals throughout the day would support high rates of muscle protein synthesis in master athletes. While additional research is warranted to confirm whether these dietary principles with a muscle-centric perspective ultimately translate into performance benefits, the ability of master athletes to age successfully allows them to leverage the lessons learned in their younger contemporaries as the foundation to fuel their future success.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
References
Areta, J.L., L.M. Burke, M.L. Ross, D.M. Camera, D.W. West, E.M. Broad, N.A. Jeacocke, D.R. Moore, T. Stellingwerff, S.M. Phillips, J.A. Hawley, and V.G. Coffey (2013). Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis. J. Physiol. 591:2319-2331.
Brady, A.J., H.M. Langton, M. Mulligan, and B. Egan (2021). Effects of eight weeks of 16:8 time-restricted eating in male middle- and long-distance runners. Med. Sci. Sports Exerc. 53:633-642.
Breen, L., K.A. Stokes, T.A. Churchward-Venne, D.R. Moore, S.K. Baker, K. Smith, P.J. Atherton, and S.M. Phillips (2013). Two weeks of reduced activity decreases leg lean mass and induces "anabolic resistance" of myofibrillar protein synthesis in healthy elderly. J. Clin. Endocrinol. Metab. 98:2604-2612.
Burd, N.A., J.W. Beals, I.G. Martinez, A.F. Salvador, and S.K. Skinner (2019). Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Sports Med. 49:59-68.
Camera, D.M., D.W. West, N.A. Burd, S.M. Phillips, A.P. Garnham, J.A. Hawley, and V.G. Coffey (2012). Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J. Appl. Physiol. 113:206-214.
Chambers, T.L., T.R. Burnett, U. Raue, G.A. Lee, W.H. Finch, B.M. Graham, T.A. Trappe, and S. Trappe (2020). Skeletal muscle size, function, and adiposity with lifelong aerobic exercise. J. Appl. Physiol. 128:368-378.
Churchward-Venne, T.A., A.M. Holwerda, S.M. Phillips, and L.J. van Loon (2016). What is the optimal amount of protein to support post-exercise skeletal muscle reconditioning in the older adult? Sports Med. 46:1205-1212.
Churchward-Venne, T.A., P.J.M. Pinckaers, J.S.J. Smeets, M.W. Betz, J.M. Senden, J.P.B. Goessens, A.P. Gijsen, I. Rollo, L.B. Verdijk, and L.J.C. van Loon (2020). Dose-response effects of dietary protein on muscle protein synthesis during recovery from endurance exercise in young men: A double-blind randomized trial. Am. J. Clin. Nutr. 112:303-317.
Coggan, A.R., R.J. Spina, M.A. Rogers, D.S. King, M. Brown, P.M. Nemeth, and J.O. Holloszy, (1990). Histochemical and enzymatic characteristics of skeletal muscle in master athletes. J. Appl. Physiol. 68:1896-1901.
Devries, M.C., L. Breen, M. Von Allmen, M.J. MacDonald, D.R. Moore, E.A. Offord, M.N. Horcajada, D. Breuille, and S.M. Phillips (2015). Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep 3:e12493.
Devries, M.C., C. McGlory, D.R. Bolster, A. Kamil, M. Rahn, L. Harkness, S.K. Baker, and S.M. Phillips (2018). Leucine, not total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. J. Nutr. 148:1088-1095.
Di Girolamo, F.G., R. Situlin, N. Fiotti, M. Tence, P. De Colle, F. Mearelli, M.A. Minetto, E. Ghigo, M. Pagani, D. Lucini, F. Pigozzi, P. Portincasa, G. Toigo, and G. Biolo (2017). Higher protein intake is associated with improved muscle strength in elite senior athletes. Nutrition 42:82-86.
Dickinson, J.M. and B.B. Rasmussen (2013). Amino acid transporters in the regulation of human skeletal muscle protein metabolism. Curr. Opin. Clin. Nutr. Metab. Care 16:638-644.
Doering, T.M., P.R. Reaburn, G. Cox, and D.G. Jenkins (2016a). Comparison of postexercise nutrition knowledge and postexercise carbohydrate and protein intake between australian masters and younger triathletes. Int. J. Sport Nutr. Exerc. Metab. 26:338-346.
Doering, T.M., P.R. Reaburn, S.M. Phillips, and D.G. Jenkins (2016b). Postexercise dietary protein strategies to maximize skeletal muscle repair and remodeling in masters endurance athletes: A review. Int. J. Sport Nutr. Exerc. Metab. 26:168-178.
Doering, T.M., P.R. Reaburn, N.R. Borges, G.R. Cox, and D.G. Jenkins (2017). The effect of higher than recommended protein feedings post-exercise on recovery following downhill running in masters triathletes. Int. J. Sport Nutr. Exerc. Metab. 27:76-82.
Fujita, S., B.B. Rasmussen, J.G. Cadenas, M.J. Drummond, E.L. Glynn, F.R. Sattler, and E. Volpi (2007). Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56:1615-1622.
Gillen, J.B., D.W.D. West, E.P. Williamson, H.J.W. Fung, and D.R. Moore (2019). Low-carbohydrate training increases protein requirements of endurance athletes. Med. Sci. Sports Exerc. 51:2294-2301.
Gorissen, S.H.M., J. Trommelen, I.W.K. Kouw, A.M. Holwerda, B. Pennings, B.B.L. Groen, B.T. Wall, T.A. Churchward-Venne, A.M.H. Horstman, R. Koopman, N.A. Burd, C.J. Fuchs, M.L. Dirks, P.T. Res, J.M.G. Senden, J. Steijns, L. de Groot, L.B. Verdijk, and L.J.C. van Loon (2020). Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. J. Nutr. 150:2041-2050.
Hamadeh, M.J., M.C. Devries, and M.A. Tarnopolsky (2005). Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise. J. Clin. Endocrinol. Metab. 90:3592-3599.
Impey, S.G., M.A. Hearris, K.M. Hammond, J.D. Bartlett, J. Louis, G.L. Close, and J.P. Morton (2018). Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 48:1031-1048.
Jeukendrup, A.E. (2017). Training the gut for athletes. Sports Med. 47:101-110.
Kato, H., K. Suzuki, M. Bannai, and D.R. Moore (2016). Protein requirements are elevated in endurance athletes after exercise as determined by the indicator amino acid oxidation method. PLoS One 11:e0157406.
Lemon, P.W., and J.P. Mullin (1980). Effect of initial muscle glycogen levels on protein catabolism during exercise. J. Appl. Physiol. 48:624-629.
Mazzulla, M., J.T. Parel, J.W. Beals, S. van Vliet, S. Abou Sawan, D.W.D. West, S.A. Paluska, A.V. Ulanov, D.R. Moore, and N.A. Burd (2017). Endurance exercise attenuates postprandial whole-body leucine balance in trained men. Med. Sci. Sports Exerc. 49:2585-2592.
Meredith, C.N., M.J. Zackin, W.R. Frontera, and J.W. Evans (1989). Dietary protein requirements and body protein metabolism in endurance-trained men. J. Appl. Physiol. 66:2850-2856.
Moore, D.R. (2014). Keeping older muscle "young" through dietary protein and physical activity. Adv. Nutr. 5:599s-607s.
Moore, D.R. (2019). Maximizing post-exercise anabolism: The case for relative protein intakes. Front. Nutr. 6:147.
Moore, D.R., T.A. Churchward-Venne, O. Witard, L. Breen, N.A. Burd, K.D. Tipton, and S.M. Phillips (2015). Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J. Gerontol. A. Biol. Sci. Med. Sci. 70:57-62.
Morton, R.W., K.T. Murphy, S.R. McKellar, B.J. Schoenfeld, M. Henselmans, E. Helms, A.A. Aragon, M.C. Devries, L. Banfield, J.W, Krieger, andS.M. Phillips (2018). A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br. J. Sports Med. 52:376-384.
Oikawa, S.Y., T.M. Holloway, and S.M. Phillips (2019). The impact of step reduction on muscle health in aging: Protein and exercise as countermeasures. Front. Nutr. 6:75.
Oikawa, S.Y., M.J. Kamal, E.K. Webb, C. McGlory, S.K. Baker, and S.M. Phillips (2020). Whey protein but not collagen peptides stimulate acute and longer-term muscle protein synthesis with and without resistance exercise in healthy older women: A randomized controlled trial. Am. J. Clin. Nutr. 111:708-718.
Phillips, S.M., S.A. Atkinson, M.A. Tarnopolsky, and J.D. MacDougall (1993). Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J. Appl. Physiol. 75:2134-2141.
Phillips, S.M., E.I. Glover, and M.J. Rennie (2009). Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J. Appl. Physiol. 107:645-654.
Smith, G.I., and B. Mittendorfer (2016). Sexual dimorphism in skeletal muscle protein turnover. J. Appl. Physiol. 120:674-682.
Snijders, T., J. Trommelen, I.W.K. Kouw, A.M. Holwerda, L.B. Verdijk, and L.J.C. van Loon (2019). The impact of pre-sleep protein ingestion on the skeletal muscle adaptive response to exercise in humans: An update. Front. Nutr. 6:17.
Stokes, T., A.J. Hector, R.W. Morton, C. McGlory, and S.M. Phillips (2018). Recent perspectives regarding the role of dietary protein for the promotion of muscle hypertrophy with resistance exercise training. Nutrients 10:180.
Stratton, M.T., G.M. Tinsley, M.G. Alesi, G.M. Hester, A.A. Olmos, P.R. Serafini, A.S. Modjeski, G.T. Mangine, K. King, S.N. Savage, A.T. Webb, and T.A. VanDusseldorp (2020). Four weeks of time-restricted feeding combined with resistance training does not differentially influence measures of body composition, muscle performance, resting energy expenditure, and blood biomarkers. Nutrients 12:1126.
Timmerman, K.L., S. Dhanani, E.L. Glynn, C.S. Fry, M.J. Drummond, K. Jennings, B.B. Rasmussen, and E. Volpi (2012). A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am. J. Clin. Nutr. 95:1403-1412.
Tinsley, G.M., J.S. Forsse, N.K. Butler, A. Paoli, A.A. Bane, P.M. La Bounty, G.B. Morgan, and P.W. Grandjean (2017). Time-restricted feeding in young men performing resistance training: A randomized controlled trial. Eur. J. Sport Sci. 17:200-207.
Williamson, E., H. Kato, K.A. Volterman, K. Suzuki, and D.R. Moore (2019). The effect of dietary protein on protein metabolism and performance in endurance-trained males. Med. Sci. Sports Exerc. 51:352-360.
Witard, O.C., S.R. Jackman, L. Breen, K. Smith, A. Selby, and K.D. Tipton (2014). Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 99:86-95.
Yang, Y., L. Breen, N.A. Burd, A.J. Hector, T.A. Churchward-Venne, A.R. Josse, M.A. Tarnopolsky, and S.M. Phillips (2012). Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. Br. J. Nutr. 108:1780-1788.