KEY POINTS
- Sustained production of muscular force and power during exercise is dependent on the generation of adenosine triphosphate (ATP) that provides energy for a number of cellular processes during muscle contraction.
- ATP is generated by non-oxidative (substrate level phosphorylation, “anaerobic”) and oxidative (oxidative phosphorylation, “aerobic”) metabolic processes, with their relative contributions being determined primarily by exercise intensity and duration.
- Fatigue often develops when substrates for ATP generation are depleted and/or when metabolic by-products accumulate in contracting muscle and the blood.
- Reductions in intramuscular levels of ATP, phosphocreatine and glycogen, and low blood glucose (hypoglycemia) can impair skeletal muscle performance. Hypoglycemia can also adversely affect central nervous system function.
- Increases in intramuscular levels of adenosine diphosphate (ADP), inorganic phosphate, magnesium and hydrogen ions and reactive oxygen species can impair muscle function. Elevated ammonia and hyperthermia may also contribute to fatigue via central and peripheral mechanisms.
- Appropriate training programs and nutritional interventions enhance fatigue resistance and exercise performance by improving the ability of skeletal muscles to sustain ATP production and resist the negative effects of metabolic by-product accumulation.
INTRODUCTION
Fatigue is a multifactorial process that reduces exercise and sports performance. It can be broadly defined as a reduction in the force or power generating capacity, or the failure to maintain the required or expected force or power output. Although fatigue involves many organ systems, most attention has focused on skeletal muscle and its ability to generate force and power. Thus, in searching for potential sites and mechanisms of fatigue, one needs to consider the steps involved in the activation of skeletal muscle during exercise. These are summarized in Figure 1 and represent sites of, or processes involved in, fatigue that are potentially affected by substrate depletion and/or accumulation of metabolic by-products.
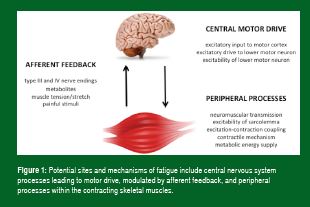
Exercise scientists have considered both central and peripheral mechanisms in the etiology of fatigue and indeed, both contribute to reduced skeletal muscle performance during exercise. Recent studies have also examined the interaction between them and have shown that activation of type III and IV afferent nerves by metabolic disturbances in contracting locomotor skeletal muscle is not only important in mediating the cardiorespiratory responses to exercise, but can also modulate central motor drive (Amann, 2011). These same afferents can be activated by metabolic disturbances in respiratory muscles, resulting in reflex sympathetic vasoconstriction in, and reduced oxygen delivery to, contracting skeletal muscles – thereby contributing to locomotor muscle fatigue (Romer & Polkey, 2008). Reductions in oxygen delivery to the brain during intense exercise may also contribute to reduced central motor drive and neuromuscular fatigue (Amann & Calbet, 2008).
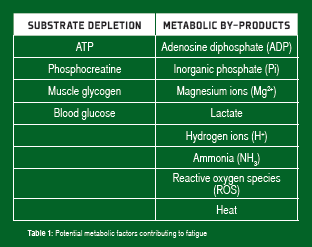
Adenosine triphosphate (ATP) is the immediate source of chemical energy for muscle contraction. Since the intramuscular stores of ATP are small (~5 mmol/kg/wet muscle), the ongoing regeneration of ATP is critical for the maintenance of force and power output during sustained exercise. At high power outputs (such as those observed during high-intensity sprint exercise), this is achieved through non-oxidative (substrate level phosphorylation, “anaerobic”) ATP production associated with the breakdown of phosphocreatine (PCr) and the degradation of muscle glycogen to lactate. At lower power outputs observed during prolonged endurance exercise, the oxidative (“aerobic”) metabolism of carbohydrates (muscle glycogen and blood glucose, derived from liver glycogen/gluconeogenesis, or the gut when carbohydrate is ingested) and lipids (fatty acids derived from intramuscular and adipose tissue triglyceride stores) provides virtually all of the ATP required for the energy-dependent processes in skeletal muscle. These metabolic processes and their importance during exercise of varying intensity and duration have been well described (Coyle, 2000; Sahlin et al., 1998). Considerable attention has focused on potential fatigue mechanisms responsible for the decline in skeletal muscle force and power output during exercise and the role of metabolic factors in fatigue. These metabolic factors can be broadly categorized as depletion of ATP and other substrates and accumulation of metabolic by-products (Table 1).
It is clear that there are multiple factors and mechanisms responsible for fatigue during exercise, and metabolic factors are just one part of a complex phenomenon. This article focuses on those metabolic factors and potentially related interventions that enhance fatigue resistance and ultimately, exercise performance.
SUBSTRATE DEPLETION
Reduced availability of ATP and key substrates involved in energy metabolism can limit ATP supply during exercise and compromise both skeletal muscle and central nervous system function. Key substrates include ATP, PCr, muscle glycogen and blood glucose.
Adenosine Triphosphate
Numerous studies have demonstrated that ATP concentration in samples of mixed muscle fibers is reasonably well protected, even during intense exercise, falling by only ~30-40% (Spriet et al., 1989). However, in analyses of single muscle fibers, ATP levels fell to a greater extent in type II (“fast”) fibers and limited the ability of these fibers to contribute to power development (Casey et al., 1996). It is also possible that small temporal and spatial reductions in ATP within the local microenvironment of the key ATP dependent enzymes (myosin ATPase, Na+/K+ ATPase, sarcoplasmic reticulum Ca2+ ATPase) and the calcium release channels of the sarcoplasmic reticulum limit these cellular processes and contribute to muscle fatigue (Allen et al., 2008). In humans, during both brief high-intensity exercise and the latter stages of prolonged, strenuous exercise, large increases in breakdown products of ATP (e.g., inosine monophosphate) imply that the rate of ATP utilization may exceed the rate of ATP resynthesis (Sahlin et al., 1998).
Phosphocreatine
The other high-energy phosphate in skeletal muscle, PCr, has a key role in rapidly generating ATP during exercise (PCr + ADP + H+ = creatine + ATP). Muscle PCr levels can be almost completely depleted after maximal exercise (Casey et al., 1996) and this contributes to a rapid reduction in muscle power output during such exercise (Sahlin et al., 1998). The recovery of muscle power-generating ability following maximal, fatiguing exercise is closely linked with the resynthesis of muscle PCr. Increased muscle PCr availability is one potential explanation for enhanced high-intensity exercise performance following dietary creatine supplementation (Casey & Greenhaff, 2000). Phosphocreatine levels may be reduced in selected muscle fibers at the point of fatigue during prolonged, strenuous exercise, coinciding with muscle glycogen depletion and the need for greater reliance on other ATP-generating pathways (Sahlin et al., 1998).
Muscle Glycogen
The association between fatigue and muscle glycogen depletion during prolonged, strenuous exercise has been observed consistently for nearly 50 years (Hermansen et al., 1967). The pioneering studies from Scandinavia informed the practice of “glycogen loading” which improves endurance exercise performance in events lasting longer than ~90 min (Hawley et al., 1997). Muscle glycogen availability may also be important for the maintenance of high-intensity exercise performance (Balsom et al., 1999). The link between muscle glycogen depletion and fatigue has been proposed to be an inability to maintain a sufficient rate of ATP resynthesis for the required power output, secondary to reduced availability of pyruvate and key metabolic intermediates within contracting skeletal muscle (Sahlin et al., 1998). Studies using electron microscopy to visualize muscle glycogen in key subsarcolemmal and inter- and intra-myofibrillar locations before and after fatiguing exercise, along with studies in isolated single fibers from rodent muscles, suggest that glycogen depletion may negatively impact sarcolemmal excitability and sarcoplasmic reticulum calcium release, resulting in fatigue (Allen et al., 2008; Ørtenblad et al., 2013). Finally, it has recently been observed that prolonged exercise results in depletion of brain glycogen stores in rats, raising the intriguing possibility that brain glycogen depletion may contribute to central fatigue (Matsui et al., 2011).
Blood Glucose
In the absence of glucose supplementation (by carbohydrate ingestion), blood glucose levels decline during prolonged, strenuous exercise, as liver glycogen becomes depleted and gluconeogenesis is unable to generate glucose at a sufficient rate. Lowered blood glucose availability (hypoglycemia) is associated with reduced rates of carbohydrate oxidation and fatigue. Increasing blood glucose by carbohydrate ingestion maintains carbohydrate oxidation, improves muscle energy balance, and enhances both endurance performance and capacity (Cermak & van Loon, 2013). Since glucose is a key substrate for the brain, hypoglycemia also reduces cerebral glucose uptake and may contribute to central fatigue (Nybo, 2003). Thus, the ergogenic benefit of carbohydrate ingestion may be partly due to improved cerebral energy balance and the maintenance of central neural drive. Improved exercise performance has also been observed after simply having carbohydrate present in the mouth and this has been linked to activation of brain centers involved in motor control (Chambers et al., 2009).
ACCUMULATION OF METABOLIC BY-PRODUCTS
Activation of the metabolic pathways that produce ATP also results in increased muscle and plasma levels of numerous metabolic by-products that potentially contribute to fatigue during exercise. These include, but are not limited to, Mg2+, ADP, inorganic phosphate Pi, H+, NH3, ROS and heat.
Mg2+, ADP, Pi
During rapid breakdown of ATP and PCr, there are increased levels of Mg2+, ADP and Pi within skeletal muscle. Increased Mg2+ can inhibit calcium release from the sarcoplasmic reticulum and impair force production, especially in combination with reduced ATP in muscle (Allen et al., 2008). Elevated ADP in muscle reduces force production and slows the rate of muscle relaxation by adversely affecting the contractile myofilaments (actin and myosin) and calcium reuptake by the sarcoplasmic reticulum (Allen et al., 2008). An increase in Pi also reduces contractile force and calcium release from the sarcoplasmic reticulum. This latter effect appears to be due to precipitation of calcium phosphate in the sarcoplasmic reticulum (Allen et al., 2008). Increases in both ADP and Pi also reduce the energy release from ATP breakdown (Sahlin et al., 1998).
Lactate, H+
Rapid breakdown of muscle glycogen during intense exercise is associated with a large increase in lactate and H+ ion production. It is generally thought that lactate itself does not have major negative effects on the ability of muscle to generate force and power, although conflicting data exists in the literature. Of greater consequence in the increase in intramuscular H+ (reduced pH or acidosis) that is associated with a high rate of ATP breakdown, non-oxidative ATP production, and the movement of strong ions (e.g., K+) across the sarcolemma. It is widely believed that increased H+ interferes with excitation-contraction coupling and force production by the myofilaments; however, in vitro studies in isolated single fibers do not always support this and even suggest that acidosis may have beneficial effects on muscle performance (Pedersen et al., 2004). Acidosis per se does not appear to impair maximal isometric force production, but does impair the ability to maintain submaximal force output (Sahlin & Ren, 1989), suggesting an inhibitory effect on muscle energy production and/or K+ homeostasis and sarcolemmal excitability. Irrespective of the underlying mechanisms, acidosis does seem to impact muscle performance since interventions that improve the ability to tolerate acidosis enhance high-intensity exercise performance. These include induced alkalosis (Costill et al., 1984) and enhanced muscle buffering capacity following high-intensity sprint training (Sharp et al., 1986) and β-alanine supplementation (Hill et al., 2007).
Ammonia and Branched Chain Amino Acids
Ammonia is produced by skeletal muscle during exercise from the breakdown of either ATP or amino acids. There is increased release of NH3 from contracting skeletal muscle and increased plasma NH3 levels during exercise. Since NH3 can cross the blood-brain barrier, this results in increased cerebral NH3 uptake and the potential to affect brain neurotransmitter levels and possibly central fatigue. More work is required to fully examine the role of NH3 in the etiology of fatigue. Of note, carbohydrate ingestion attenuates plasma and muscle NH3 accumulation during prolonged exercise (Snow et al., 2000) and this may be another mechanism by which carbohydrate ingestion exerts its ergogenic effect.
Although the “central fatigue hypothesis” proposed by the late Prof. Eric Newsholme still remains largely a theoretical construct, the potential interactions between the metabolism of branched-chain amino acids (BCAA – leucine, isoleucine and valine), cerebral tryptophan uptake, and brain serotonin levels during prolonged exercise have been implicated in central fatigue. Tryptophan is a serotonin precursor and cerebral tryptophan uptake is related to both the plasma concentration of free tryptophan and the free tryptophan/BCAA ratio. During exercise, a fall in plasma BCAA levels and an increase in free tryptophan can increase tryptophan uptake by the brain and increase serotonin levels and central fatigue. BCAA ingestion has been proposed for attenuating the development of central fatigue by maintaining plasma BCAA levels and blunting cerebral tryptophan uptake, but this does not appear to be effective. A better strategy may be to ingest carbohydrate which blunts the exercise-induced rise in plasma fatty acid levels. Since fatty acids and tryptophan compete for binding sites on plasma albumin, the lower fatty acid level associated with carbohydrate ingestion attenuates the rise in the free tryptophan/BCAA ration (Davis et al., 1992).
Reactive Oxygen Species
During exercise, ROS such as superoxide anions, hydrogen peroxide and hydroxyl radicals are produced within contracting skeletal muscle. At low levels, ROS act as important signaling molecules; however, their accumulation at higher levels can negatively affect a number of processes involved in the generation of muscle force and power and induce fatigue (Allen et al., 2008; Ferreira Reid, 2008). There are several enzymatic antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase) within skeletal muscle that degrade ROS, and non-enzymatic antioxidants, such as reduced glutathione, β-carotene, and vitamins C and E, that can also counteract the negative effects of ROS. Administration of N-acetylcysteine enhances muscle antioxidant capacity and is associated with reduced muscle fatigue and enhanced endurance cycling performance (Ferreira & Reid, 2008). Studies with vitamin C and E supplementation are somewhat equivocal (Powers et al., 2011), but skeletal muscle antioxidant enzyme activities are increased by exercise training.
Heat
Only ~20% of the oxygen consumption during exercise is converted to mechanical work, with the remainder of the energy released as heat, a major by-product of metabolism. Much of this heat is dissipated via the evaporation of sweat and other heat loss mechanisms. However, when the rate of heat production is high, as during strenuous exercise, and/or when heat loss is compromised by increased environmental heat and/or humidity, there can be a significant increase in core and tissue temperatures (hyperthermia). Hyperthermia can affect both central and peripheral processes involved in muscle fatigue (Nybo, 2008) and in extreme cases may be fatal. The negative effects of hyperthermia on exercise performance are potentiated by dehydration that results from large sweat-induced fluid losses (González-Alonso et al., 1997). Strategies to minimize the negative effects of hyperthermia include heat acclimatization, pre-exercise cooling, and fluid ingestion.
PRACTICAL APPLICATIONS
- Physical training increases fatigue resistance by enhancing maximal oxygen uptake, due to increased maximal cardiac output and muscle capillary density and oxidative capacity; increasing the lactate threshold, with implications for rates of muscle glycogen utilization; and increasing muscle buffer capacity and improved electrolyte, notably K+, regulation.
- Nutritional interventions that alter carbohydrate and protein availability can modulate training adaptations (Hawley et al., 2011).
- Given the carbohydrate dependence during intense training and competition, strategies to enhance carbohydrate availability and maximize carbohydrate oxidation, such as muscle glycogen loading and carbohydrate ingestion, are effective in enhancing exercise performance.
- Induced alkalosis can enhance high intensity sprint performance, as does β-alanine supplementation by increasing muscle buffer capacity. Dietary creatine supplementation enhances the ability to repeat high-intensity efforts.
- Fluid ingestion, heat acclimatization and pre-cooling are effective strategies to attenuate the exercise-induced hyperthermia.
SUMMARY
Increased non-oxidative and oxidative ATP production via metabolic pathways in contracting skeletal muscle is essential for the maintenance of force and power output during exercise. However, substrate depletion and metabolic by-product accumulation are potential causes of fatigue, by impairing both central neural and peripheral processes involved in muscle activation. Reduced PCr availability can limit power production during high-intensity sprint exercise, whereas carbohydrate depletion is a major limitation to endurance performance. During intense exercise, increased Pi and H+ may contribute to fatigue, and during prolonged, strenuous exercise, the accumulation of NH3, ROS and heat can limit performance. Appropriate training programs and nutritional interventions are strategies to enhance fatigue resistance and enhance exercise performance.
REFERENCES
- Allen, D.G., G.D. Lamb, and H. Westerblad (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol. Rev. 88: 287-332.
- Amann, M (2011). Central and peripheral fatigue: interaction during cycling exercise in humans. Med. Sci. Sports Exerc. 43: 2039-2045.
- Amann, M. and J.A.L. Calbet (2008). Convective oxygen transport and fatigue. J. Appl. Physiol. 104: 861-870.
- Balsom, P.D., G.C. Gaitanos, K. Söderlund, and B. Ekblom (1999). High-intensity exercise and muscle glycogen availability in humans. Acta Physiol. Scand. 165: 337-345.
- Casey, A., D. Constantin-Teodosiu, S. Howell, E. Hultman, and P.L. Greenhaff (1996). Metabolic responses of type I and II muscle fibres during repeated bouts of maximal exercise in humans. Am. J. Physiol. 271: E38-E43.
- Casey, A., and P.L. Greenhaff (2000). Does dietary creatine supplementation play a role in skeletal muscle metabolism and performance. Am. J. Clin. Nutr. 72: S607-S617.
- Cermak, N.M., and L.J.C. van Loon (2013). The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 43: 1139-1155.
- Chambers, E.S., M.W. Bridge, and D.A. Jones (2009). Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. J. Physiol. 587: 1779-1794.
- Costill, D.L., F. Verstappen, H. Kuipers, E. Janssen, and W. Fink (1984). Acid-base balance during repeated bouts of exercise: influence of HCO3-. Int. J. Sports Med. 5: 228-231.
- Coyle, E.F. (2000). Physical activity as a metabolic stressor. Am. J. Clin. Nutr. 72: S512-S520.
- Davis, J.M., S.P. Bailey, J. Woods, F. Galiano, M. Hamilton, and W. Bartoli (1992). Effects of carbohydrate feedings on plasma free-tryptophan and branched-chain amino acids during prolonged cycling. Eur. J. Appl. Physiol. 65: 513-519.
- Ferreira, L.F., and M.B. Reid (2008). Muscle-derived ROS and thiol regulation in muscle fatigue. J. Appl. Physiol. 104: 853-860.
- González-Alonso, J., R. Mora-Rodriguez, P.R. Below and E.F. Coyle (1997). Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. J. Appl. Physiol. 82: 1229-1236.
- Hawley, J.A., E.J. Schabort, T.D. Noakes, and S.C. Dennis (1997). Carbohydrate-loading and exercise performance. Sports Med. 24: 73-81.
- Hawley, J.A., L.M. Burke, S.M. Phillips and L.L. Spriet (2011). Nutritional modulation of training-induced skeletal muscle adaptations. J. Appl. Physiol. 110: 834-845.
- Hermansen, L., E. Hultman, and B. Saltin (1967). Muscle glycogen during prolonged severe exercise. Acta Physiol. Scand. 71: 129-139.
- Hill, C.A., R.C. Harris, H.J. Kim, B.D. Harris, C. Sale, L.H. Boobis, C.K. Kim, and J.A. Wise (2007). Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids. 32: 225-233.
- Matsui, T., S, Soya, M. Okamoto, Y. Ichitani, K. Kawanaka, and H. Soya (2011). Brain glycogen decreases during prolonged exercise. J. Physiol. 589: 3383-3393.
- Nybo, L. (2003). CNS fatigue and prolonged exercise: effect of glucose supplementation. Med. Sci. Sports Exerc. 35: 589-594.
- Nybo, L. (2008). Hyperthermia and fatigue. J. Appl. Physiol. 104: 871-878.
- ·Ørtenblad, N., H. Wetserbald, and J. Nielsen (2013). Muscle glycogen stores and fatigue. J. Physiol. 591: 4405-4413.
- Pedersen, T.H., O.B. Nielsen, G.D. Lamb and D.G. Stephenson (2004). Intracellular acidosis enhances the excitability of working muscle. Science. 305: 1144-1147.
- Powers, S., W.B. Nelson and E. Larson-Meyer (2011). Antioxidant and vitamin D supplements for athletes: sense of nonsense? J. Sports Sci. 29: S47-S55.
- Romer, L.M. and M.I. Polkey (2008). Exercise-induced respiratory muscle fatigue: implications for performance. J. Appl. Physiol. 104: 879-888.
- Sahlin, K., and J-M. Ren (1989). Relationship of contraction capacity to metabolic changes during recovery from fatiguing contraction. J. Appl. Physiol. 67: 648-654.
- Sahlin, K., M. Tonkonogi, and K. Söderlund (1998). Energy supply and muscle fatigue in humans. Acta Physiol. Scand. 162: 261-266.
- Sharp, R.L., D.L. Costill, W.J. Fink, and D.S. King (1986). Effects of eight weeks of bicycle ergometer sprint training on human muscle buffering capacity. Int. J. Sports Med. 7: 13-17.
- Snow, R.J., M.F. Carey, C.G. Stathis, M.A. Febbraio, and M. Hargreaves (2000). Effect of carbohydrate ingestion on ammonia metabolism during exercise in humans. J. Appl. Physiol. 88: 1576-1580.
- Spriet, L.L., M.I. Lindinger, R.S. McKelvie, G.J.F. Heigenhasuer and N.L. Jones (1989). Muscle glycogenolysis and H+ concentration during maximal intermittent cycling. J. Appl. Physiol. 66: 8-13.