Sports Science Exchange 90
VOLUME 16 (2003) NUMBER 3
Diabetes, Exercise and Competitive Sports
Peter A. Farrell, Ph.D.
Department of Exercise and Sport Science
East Carolina University
Greenville, NC 27858
KEY POINTS
- People with diabetes mellitus—rapidly approaching one-third of the US population—either cannot produce insulin (Type 1 DM) or the insulin they produce is ineffective in stimulating the uptake of blood sugar (glucose) into the body’s cells (Type 2 DM). Accordingly, if diabetes is untreated, blood sugar rises to dangerously high levels that can eventually cause blindness, nerve damage, and other complications.
- Blood sugar can be controlled by the appropriate administration of insulin and other drugs and/or by the manipulation of dietary carbohydrate and exercise.
- During exercise, the contracting muscles produce their own insulin-like effect, causing the rapid uptake of glucose from the blood. In people without diabetes, the body naturally reduces its production of insulin to compensate; otherwise, blood glucose would fall precipitously. (A low blood glucose concentration is known as hypoglycemia.) Those with Type 1 DM (and those with Type 2 DM who use insulin to control their blood sugar) must adjust their pre-exercise insulin dosage and their carbohydrate intake before, during, and after exercise to avoid becoming hypoglycemic.
- Regular exercise training is usually beneficial for those with diabetes because exercise can reverse many of the adverse metabolic effects of the disease, including the likelihood of becoming obese.
- Although precautions must be taken, athletes with uncomplicated diabetes (no other serious diseases) have become champions at elite levels in a wide variety of sports.
INTRODUCTION
Regular exercise is highly recommended for many people who have either Type 1 or Type 2 Diabetes Mellitus (DM). People with diabetes must use extra vigilance in preparing for exercise because they lack insulin (Type 1 DM) or because the insulin they have is defective in its ability to stimulate glucose uptake (Type 2 DM). Normal production and action of insulin are critical to a "correct" metabolic response to exertion. The person with diabetes can, however, reach marvelous levels of physical accomplishment, and outstanding examples of this fact are found in most college, professional and Olympic sports. One of the most impressive of these athletes is Sir Steven Redgrave, winner of gold medals in rowing for Great Britain at five successive Olympic Games from 1984 to 2000. He was diagnosed with diabetes about two years before the Sydney 2000 Olympic Games.
Defining the Disease
Type 1 DM is characterized by an autoimmune destruction of pancreatic beta cells, i.e., the body mistakenly destroys the very tissues that make and secrete insulin. While insulin has many functions in the body, five are particularly important during or after exercise:1) stimulation of glucose uptake into most cells of the body, 2) inhibition of glucose release from the liver, 3) inhibition of the release of fatty acids from storage depots, 4) facilitation of protein synthesis in the body’s cells, and 5) stimulation of the resynthesis of muscle glycogen after exercise.
Type 2 DM is very different from Type I DM; insulin is present, but it does not function efficiently to stimulate glucose uptake into cells (termed "insulin resistance"). The body tries to compensate for this defect by secreting more and more insulin, but eventually the reserve capacity of the pancreatic beta cells declines and the glucose concentration in the blood rises. Both Type 1 and 2 DM are diagnosed by detection of a fasting (>8 h) plasma glucose level that exceeds 126 mg/dl or a plasma glucose concentration greater than 200 mg/dl at 2 h after an oral glucose challenge of 75 g of glucose or by the appearance of other classic symptoms of diabetes. It is standard practice to follow the initial diagnosis by more extensive and repeated testing.
RESEARCH REVIEW
Metabolic Responses to Acute Exercise
In contrast to most hormones, concentrations of insulin in the blood decline during exercise in people without diabetes because less insulin is secreted from the pancreas. Given that skeletal muscle is quantitatively the most important tissue in the body for glucose uptake, especially during exercise, and given the fact that insulin is the primary stimulus for glucose uptake into resting cells, this decline in insulin secretion during exercise at first seems paradoxical. However, the insulin requirement for glucose uptake diminishes during exercise, because muscle contractions per se stimulate glucose uptake into muscle even when insulin is absent (Hayashi et al., 1997; Holloszy, 2003; Nesher et al., 1985; Ploug et al., 1984). The natural decline in insulin during exercise is necessary to prevent hypoglycemia.
For people with Type 1 DM who appropriately manage their blood glucose levels and have adjusted their pre-exercise insulin dose, the fuels used during exercise are not markedly different from fuels used by nondiabetics, as long as the exercise intensity is moderate (Raguso et al., 1995; Wahren, 1979). The normal decrease in blood insulin during exercise in people without diabetes and in those with Type 2 DM allows the two most important fuels for exercise, carbohydrates and fats, to be mobilized and used by muscle. High concentrations of insulin suppress the ability of the liver to release glucose and deliver it into the plasma. Elevated insulin also inhibits the release of fatty acids into the blood from adipose tissue and perhaps from fat stored in muscle. Unfortunately, the normal decline in insulin cannot occur in people with Type 1 DM because they cannot produce and therefore cannot decrease insulin production. Thus, the prevailing concentration of blood insulin depends on the timing of the last insulin injection (or infusion rate for those who use an insulin pump). Consequently, the ability to mobilize fat and carbohydrate fuels for exercise may be compromised in people with diabetes. Insulin in the blood must be at low levels during exercise, but maintaining at least some circulating insulin is an absolute requirement for other aspects of exercise metabolism that are discussed below.
Key Concept for Type 1 DM—Over- and Under-Insulinization
The following comments pertain to people with Type 1 DM or those with Type 2 DM who must use insulin to control their blood glucose.
Over-insulinization, i.e., the administration of too much insulin to control blood glucose, is best considered relative to the conditions at the insulin target tissues (Wasserman et al., 2002). In the case of exercise, the most critical insulin target tissue is skeletal muscle, and muscle requires less insulin during exercise. Accordingly, for brief, moderate-intensity exercise, a reduction of approximately 50% in the pre-exercise insulin dose is warranted in many cases (Schiffrin & Parikh, 1985). If prolonged exercise (>90 min) is anticipated, a 70–80% reduction of insulin may be needed to avoid a fall in blood glucose to a dangerous level (hypoglycemia). Even with what appears to be an appropriate reduction in the pre-exercise insulin dose, over-insulinization can still occur because contractions make the muscle more sensitive to insulin. Compounding the issue further, exercise increases muscle blood flow and heat production, both of which may enhance the absorption of injected insulin. An overarching guideline is that the dose of insulin administered before exercise should be reduced. The amount of that reduction should take into account many factors, such as training status, time of exercise after the last meal, intensity and duration of exercise, and the extent to which the activity to be performed that day is habitual or unaccustomed.
Under-insulinization can result in excessive concentrations of blood glucose (hyperglycemia) during exercise because very low insulin concentrations are insufficient to inhibit glucose release from the liver. A documented consequence of starting exercise with too little insulin (with plasma glucose >270 mg/dl) is an even greater hyperglycemia during exercise (Berger et al., 1977). This concern is probably more pertinent to brief, high-intensity exercise such as occurs in many athletic contests as opposed to prolonged moderate-intensity exercise. Additionally, other hormones, especially glucagon secreted by the pancreas and epinephrine (adrenaline) from the adrenal glands, become more effective in stimulating glucose production when insulin is too low (Cryer, 2001).
During low-intensity or prolonged exercise, fatty acids become an important source of energy for the active muscle. Overinsulinization will inhibit fatty acid release from fat stores, whereas underinsulinization will allow an excessive mobilization of fatty acids, which can cause ketone production and release by the liver, a condition called diabetic ketosis. (Ketones are acids that markedly increase the acidity of body fluids and thus must be avoided.)
It is impossible to provide a single set of guidelines appropriate for all people with diabetes who wish to exercise, and the best advice is to encourage diabetics to document for themselves what works and what does not work (Wallberg-Henriksson, 1989). (All adjustments of insulin dose prior to exercise must be made relative to carbohydrate ingestion, as will be discussed later.)
The person with Type 1 DM can become quite proficient in simulating an exercise-induced decline in circulating insulin by decreasing the amount of insulin injected or infused before exercise begins. Avoiding hypoglycemia or hyperglycemia can also be achieved by increasing or decreasing, respectively, the amount of carbohydrate ingested before planned exercise. Such adjustments in carbohydrate intake are the only alternative for unplanned exercise when the amount of circulating insulin is set by the prior injection or pump infusion rate. This point has special meaning for children because their daily physical activities are often spontaneous. Currently, we know very little about the metabolic adjustments to exercise in children with diabetes (Campaigne et al., 1984; Dahl-Jørgensen et al., 1980; Ludvigsson, 1980;). Children with diabetes should be encouraged to participate unstructured physical activity and in organized sports, and the recommendations in this article can serve as beginning guides for glucose management for both children and adults.
Signs and Symptoms of Hypoglycemia and Hyperglycemia
Parents, friends, coaches, athletic trainers, sports nutritionists, and sports team members should know the typical signs of hypo and hyperglycemia. Unfortunately, many of theses signs and symptoms are similar to typical responses to acute exercise in nondiabetic populations.
Symptoms of hypoglycemia: Normal plasma glucose concentrations in overnight-fasted people are usually in the range of 80–100 mg/dl (4.4–5.5 mM). There are exceptions, but most individuals with fasted glucose levels below 45 mg/dl (2.5 mM) are considered hypoglycemic. Sweating, pounding heart, shaking, hunger, confusion, drowsiness, difficulty with speech, incoordination, nausea, and headache. In children, irritability and temper tantrums are also symptoms of hypoglycemia.
Symptoms of hyperglycemia: Fasted plasma glucose concentrations above 110 mg/dl (6.1 mM) are often considered hyperglycemic levels. Typical symptoms of hyperglycemia are less standard than for hypoglycemia. During an acute episode of hyperglycemia, the person can experience restlessness or nervousness. Longer-term hyperglycemia leads to thirst, fatigue, muscle cramps, blurred vision, nausea, drowsiness, and abdominal pain.
Resistance Exercise For People With Type 1 DM
The guidelines and principles provided by the American Diabetes Association (2002) and other organizations are primarily based on literature specific to endurance (aerobic) exercise. In general, people with Type 2 DM who perform chronic resistance exercise gain the same metabolic and muscle hypertrophy benefits as do nondiabetics. It is quite disheartening, however, that only three studies (Durak et al., 1990; Mandroukas et al., 1986; Mosher et al., 1998) have investigated the effects of resistance exercise on people with Type 1 DM, and of those, only one (Durak et al., 1990) included an exercise protocol that used solely resistance exercise. The three studies focused on strength gains, the blood lipid profile, body composition, and/or regulation of plasma glucose, but none reported blood pressure responses to acute resistance exercise or to resistance training. This is a major shortcoming because there is some indication that people with Type 1 DM have higher systolic and diastolic blood pressures during bicycle and handgrip exercise compared to nondiabetic control subjects (Nazar et al., 1975; Christensen et al., 1984; Torffvit et al., 1987; Newkumet et al., 1994). Heavy exercise of all types, but particularly resistance exercise, increases arterial pressure to very high levels in people without diabetes. Such high pressures could damage the blood vessels in the eyes of those with diabetes. Therefore, until resistance exercise is proven harmless, the person with diabetes who has preexisting retinal damage should avoid this type of exercise. It must be realized, however, that no published data exist demonstrating that resistance exercise of any intensity causes damage to blood vessels in the eyes or elsewhere.
People with diabetic autonomic neuropathies have difficulty controlling blood pressure, cardiac output, and distribution of blood flow. Each of these could compromise the ability to exercise, especially when such exercise is strenuous. Those seeking practical advice on this topic should refer to an article published by Hornsby (1990).
In summary, the benefits of resistance exercise for people with Type 1 DM are not yet established. Because of its potential to develop and maintain muscle mass, this form of exercise should be aggressively studied from a risks/benefit perspective. One might speculate that the benefits of a correctly designed program of resistance exercise would far exceed the risks to adequately screened people with Type 1 DM.
Benefits Of Regular Exercise For Diabetics
As far as body weight and composition are concerned, the benefits of participation in regular physical activity are not entirely the same for those with Type 2 DM and those with Type 1 DM. As one example, there is a strong tendency, fostered by a genetic predisposition, for people with Type 2 DM—but not Type 1 DM—to become obese. One potential positive outcome of regular exercise is that the extra energy expended during and after exercise can help people with Type 2 DM gain control over excessive weight gain. However, weight loss due to exercise alone is usually not substantial, and exercise is more effective for this outcome when coupled with a reduced-calorie diet.
Because insulin is needed for the maintenance of muscle mass, people with Type 1 DM may increase their relative accumulation of body fat while total body weight remains constant.
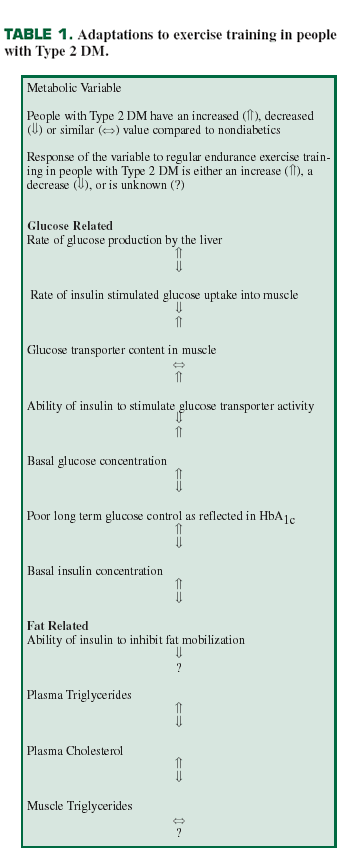
The benefits of regular endurance exercise for people with Type 2 DM are numerous, well supported by the literature, and prove the value of exercise for both preventing (Eriksson & Lindgarde, 1991; Helmrich et al., 1991; Knowler et al., 2002; Pan et al., 1997; Tuomilehto et al., 2001) and treating (Rogers, 1989) this disease. Table 1 lists adaptations to exercise training for Type 2 DM. The important message from Table 1 is that a program of regular physical activity can reverse many of the defects in metabolism of both glucose and fat that occur in people with Type 2 DM. Unfortunately, because there is a lack of similar specific information on adaptations to exercise training for those with Type 1DM, a similar table for Type 1 DM cannot be constructed. However, it is known that people with Type 1 DM typically live longer if they participate in regular physical activity as a part of their lifestyles (Moy et al., 1993).
Exercise and Hemoglobin A1c. Hemoglobin A1c (HbA1C) is used as an index of long-term blood glucose control, i.e., glucose levels that have existed for the previous 2–3 months. The lower the value for HbA1c, the better. The fact that HbA1c is reduced by chronic exercise in people with Type2 DM is important because this means that long-term glucose control has been realized and that with "better" control comes reduced risks for complications. Unfortunately, the same cannot be said for Type 1 DM. Many studies (Bævre et al., 1985; Horton, 1996; Laaksonen et al., 2000; Landt et al., 1985; Wallberg-Henriksson et al., 1984, 1986; Zinman et al., 1984)show that although other defects in metabolism can be reduced by chronic exercise in people with Type 1 DM, long-term glucose control as measured by HbA1c is not changed. This finding could, however, be a function of limitations in the literature. As one example, many studies showing no change in HbA1c used training protocols that lasted only 1–2 months, but changes in HbA1c do not stabilize at lower levels until at least 80 days after normalization of plasma glucose concentrations caused by insulin treatment. Moreover, other studies (Huttunen et al., 1989; Perry et al., 1997) suggest that better glucose regulation is found after chronic exercise in those with Type 1 DM. Another difficulty in interpretation of the previous literature on the effects of exercise on glucose control in Type 1DM is the dearth of information about the duration of diabetes affliction in the subjects studied. One might speculate that people who have had diabetes for decades may be less responsive to exercise treatments when compared to newly diagnosed individuals.
Psychological benefits of regular exercise are well established for people without diabetes. Such benefits in all probability also occur in people with diabetes, but very few studies have addressed this question. It is possible that the added vigilance in glucose monitoring required for safe exercise could act has a positive reinforcement for people with diabetes to better monitor their blood glucose levels. Positive alterations in mood or psychological state such as anxiety reduction, increase in vigor, and improved sense of self-worth would obviously be helpful for diabetic patients in "handling" their disease and could also have a positive impact on athletic performance.
The Diabetic Athlete
Athletes with Type I DM who have achieved superior levels of performance have established patterns of carbohydrate feedings and insulinization that work for them. It is clear that individualized trial-and-error with manipulations of diet and insulin administration must occur if such an athlete is to establish reliable glucose control.
The attention of the public is usually drawn to athletes who have succeeded in spite of Type 1 DM, perhaps because we better appreciate their having overcome a lifelong affliction with the disease, in contrast to athletes with Type 2 DM, whose disease typically is manifested in adulthood. In fact, it is unclear how many top athletes have Type 2 DM, perhaps because extensive regular exercise ameliorates insulin resistance to such a great extent that the budding athlete with latent or prior Type 2 DM simply overcomes the disease to the point that it is not apparent. While purely speculation at this time, it is conceivable that the accomplished athlete with persistent Type 2 DM is a rare find.
For the most part, previous studies on the metabolic response to exercise in diabetic subjects used exercise protocols that did not reflect sports conditions. Most sports require short periods of very high intensity effort, and most of what we know about exercise metabolism in diabetes is based on research that employed prolonged, moderate and constant-intensity exercise. Thus, for people with diabetes, the metabolic demands of, and acute responses to, participation in most sports are largely unknown (Peirce, 1999). Another major gap in our knowledge is a lack of understanding of how diabetes affects recovery from athletic contests. Our personal observations suggest that diabetic university-level athletes in football, swimming, and track may not recover as rapidly as their nondiabetic teammates. This might be because the postexercise resynthesis of glycogen, the storage form of glucose in the muscle and liver is slower in people with diabetes (Hermansen, 1980). Thus, these athletes may not be completely glycogen-restored before the next practice or game. Another documented concern is delayed hypoglycemia. This phenomenon can occur for from 4–48 hours after exercise (MacDonald, 1987). Delayed hypoglycemia that occurs at night can disturb sleep, which could contribute to prolonged fatigue during the training season.
The athletic team support staff should know where their athletes who have diabetes store their insulin and syringes. They should also have a source of simple carbohydrates readily available for its diabetic athletes. Glucose tablets and sports drinks containing carbohydrates and electrolytes work well. Some insulin pump-treated diabetic athletes who participate in contact sports prefer to remove the pump during practice or games and this requires obvious precautions in terms of securing the pump from tampering by others, protection from theft, and inadvertent damage on the sidelines.
Sport-Specific Considerations
Some sports present greater challenges to the diabetic athlete than do others (Peirce, 1999). For example, because of unforeseen logistical considerations in competition, track-and-field events may occur earlier or later than planned, and this could disrupt the athlete’s strategies for insulinization and carbohydrate intake. Likewise, it is difficult to predict when an American football player will be required to exert high levels of energy expenditure over the 2–3 hour time period for a typical game. Moreover, winter sports for Type 1 diabetics present the added consideration that insulin in the pump or that being carried for injections may freeze. Also, protection of the insulin pump from damage is a concern in any contact sport, including football, rugby, lacrosse, ice or field hockey. Although scuba diving was once contraindicated for people with Type 1 DM, is it now clear that with proper training this activity can be enjoyed (Harper, 2002). Because of potential damage to the retina, such sports as boxing, judo, and karate should be entered into only after careful consideration of the risks involved and after proper medical clearance. Still, athletes with diabetes are the ones who should ultimately decide in which sports they will participate and how intense they will exert themselves in the chosen sport.
Dealing With Complications Of The Disease
The extra vigilance needed for gaining metabolic control during exercise should be extended to special considerations dictated by complications of diabetes (Skyler, 1998). People with long-standing and/or poorly controlled diabetes can suffer from hypertension, neuropathies (damage to nerves), retinopathy (damage to the blood vessels in the eyes that can lead to blindness), kidney damage, heart disease, and a higher frequency of foot ulcers.
Hypertension. Because hypertension and cardiovascular disease are more common in people with diabetes, beta blockers (drugs block the action of adrenaline) are often prescribed for such conditions, and such medications can alter the metabolic response to exercise (Gittoes et al., 1997). As an example, those taking beta blockers may be at greater risk for developing hypoglycemia because adrenaline is needed to stimulate glucose mobilization from the liver into the blood during exercise.
Neuropathies. Diabetic patients with cardiovascular autonomic neuropathy have reduced maximal heart rates and cardiac outputs. Thus, exercise prescriptions based on normal values would grossly overextend the diabetic patient (Waxman & Nesto, 2002). Additionally, people with such neuropathies may be more prone to undisclosed heart disease (Gu et al., 1998). Therefore, the medical evaluation of the diabetic athlete prior to starting an exercise program must be comprehensive so that all such conditions are detected. People with diabetic neuropathies may also be at a disadvantage from a balance and coordination standpoint because the activation of muscle spindles (nerve receptors in the muscle that sense changes in muscle length) may be at least partially compromised (Vinik & Erbas, 2002).
Retinal Concerns. Diabetic retinopathy is more likely in a given patient as the disease progresses. One of the concerns diabetologists have about any strenuous exercise in this population is that significant elevations in blood pressure may damage already weakened blood vessels, especially in the eyes. The logic concerning the avoidance of strenuous exercise in patients with moderateto- severe diabetic retinopathy is clinically appropriate yet scientifically unsubstantiated because of the lack of formal studies. No studies exist which have evaluated the eye vasculature before and after acute or chronic resistance exercise. Prior to commencing an exercise program, it is advisable to have a complete eye examination if there is any concern about the current status of the retinae. Many people with Type 1 DM perform resistance exercise on a regular basis, but little research has been done on the outcomes of such exercise.
Foot Ulcers. Finally, sensory loss can occur with long-term and/or poorly controlled diabetes, and this dictates greater vigilance in inspecting one’s feet for the presence of ulcers, especially in sports where repetitive pounding of the feet can occur. Obese Type 2 diabetics are advised to engage in activities such as water sports or cycling that do not place repetitive stress on the feet and joints as occurs in running. The greater availability of recumbent bi- and tri-cycles should encourage overweight individuals (regardless of diabetic status) to enjoy this form of exercise/transportation. Some extra caution is probably wise for the older person who has had Type 1 or 2 DM for many years. Still, one report on a small number of people with Type 1 DM suggests that even longstanding Type 1 DM (10–29 y) does not significantly alter the metabolic, endocrine and cardiorespiratory response to acute exercise as long as the patients are in good glucose control and have no diabetes-associated complications (Nugent et al., 1997).
SUMMARY
1. People with either Type 1 or Type 2 DM can reach very high levels of athletic performance. Once they reach this level of accomplishment, they have learned how to coordinate their carbohydrate and/or insulin administration regimens so that they can compete without severe changes in blood glucose concentrations. For the recreational or beginning exerciser, there will be a period of trial-and-error because the ideal quantity and timing of insulinization and carbohydrate supplementation is highly individualistic.
2. Better glucose control is gained by reducing the pre-exercise dose of insulin by 50–80%, depending on the type, duration, and intensity and familiarity of exercise.
3. The benefits of regular exercise in people with diabetes are similar to those in persons without the disease—as long as the diabetic is in good glucose control and has no major complications of the disease. Those benefits outweigh potential problems caused by the metabolic stress of exercise, providing that proper medical screening has occurred.
4. Resistance exercise for people with Type 1 DM is becoming popular and is probably appropriate. However, current recommendations should be based on "best clinical judgment" because of the absence of data from controlled scientific studies.
REFERENCES
American Diabetes Association (2002). Clinical Practice Recommendations:2002. Diabetes Care 25 (suppl. 1):S64–S68.
Bævre, H., O. Søvik, A. Wisnes, and E. Heiervang (1985). Metabolic responses to physical training in young insulin-dependent diabetics. Scand. J. Clin. Lab. Invest. 45:109–114.
Berger, M., P. Berchtold, H.J. Cuppers, H. Drost, H.K. Kley, W.A. Muller, W. Wiegelmann, H. Zimmerman-Telschow, F.A. Gries, H.L. Kruskemper, and H. Zimmermann (1977). Metabolic and hormonal effects of muscular exercise in juvenile type diabetics. Diabetologia 13:355–365.
Campaigne, B.N., T.B. Gilliam, M.L. Spencer, R.M. Lampman, and M.A. Schork (1984). Effects of a physical activity program on metabolic control and cardiovascular fitness in children with insulin-dependent diabetes mellitus. Diabetes Care 7:57–62.
Christensen, N.J., H. Galbo, A. Gjerris, J.H. Henriksen, J. Hilsted, M. Kjaer, and H. Ring-Larsen (1984). Whole body and regional clearances of noradrenaline and adrenaline in man. Acta Physiol. Scand. 527:17–20.
Cryer, P.E. (2001). The prevention and correction of hypoglycemia. In: L.S. Jefferson and A.D. Cherrington (eds.) Handbook of Physiology, vol. 2. Oxford:Oxford University Press, pp.1057–1093.
Dahl-Jørgensen, K., H.D. Meen, K.F. Hanssen, and O. Aagenaes (1980). The effect of excercise on diabetic control and hemoglobin A1 in children. Acta Paediatr. Scand. 283:53–56.
Durak, E.P., L. Jovanovic-Peterson, and C.M. Peterson (1990). Randomized crossover study of effect of resistance training on glycemic control, muscular strength and cholesterol in type I diabetic men. Diabetes care 13:1039–1043.
Eriksson, K., and F. Lindgarde (1991). Prevention of Type 2 (noninsulin dependent diabetes) diabetes mellitus by diet and physical exercise. Diabetalogia 34:891–898.
Gittoes, N.J.L., M.J. Kendall, and R.E. Ferner (1997). Drugs and diabetes mellitus. In: J.C. Pickup and G. Williams (eds.) Textbook of Diabetes, 2nd ed. Oxford:Blackwell Science, pp. 69.1–69.12.
Knowler W.C., E. Barrett-Connor, S.E. Fowler, R.F. Hamman, J.M. Lachin, E.A. Walker, and D.M. Nathan; Diabetes Prevention Program Research Group (2002). Reduction in the incidence of Type 2 Diabetes with lifestyle intervention or metformin. N. Eng. J. Med. 346:393–403.
Gu, K., C. Cowie, and M.I. Harris (1998). Mortality in adults with and without diabetes in a national cohort of the U.S. population. Diabetes Care 21:1138–1145.
Harper, P. (2002). The diabetic athlete as a role model. In: N.B. Ruderman, J.T. Devlin, S.H. Schneider, and A. Kriska (eds.) Handbook of Exercise in Diabetes. Alexandria, VA:American Diabetes Association, pp.615–624.
Hayashi, T., J.F.P. Wojtaszewski, and L.J. Goodyear (1997). Exercise regulation of glucose transport in skeletal muscle. Am. J. Physiol. (Endocrinol. Metab.) 273:E1039–E1051.
Helmrich, S., D.R. Ragland, R.W. Leung, and R.S. Paffenbarger, Jr. (1991). Physical activity and reduced occurence of non-insulin dependent diabetes mellitus. N. Eng. J. Med. 325:147–152.
Hermansen, L. (1980). Resynthesis of muscle glycogen stores during recovery from prolonged exercsie in non-diabetic and diabetic subejcts. Acta Paediatr. Scand. 283:33–38.
Holloszy, J. O. (2003). A forty-year memoir of research on the regulation of glucose transport into muscle. Am. J. Physiol. (Endocrinol. Metab.) 284:E453–467.
Hornsby, G. (1990). Putting a lift in your workout. DiabetesForecast 43 (Jan.):55–60.
Horton, E. S. (1996). Exercise for the patient with insulin dependent diabetes mellitus. In: D. LeRoith, S.I. Taylor, and J.M. Olefsky (eds.) Diabetes Mellitus. Philadelphia:Lippincott-Raven, pp. 395–402.
Huttunen, N.P., S.L. Lankela, M. Knip, P. Lautala, M.L. Kaar, K. Laasonen, and R. Puukka (1989). Effect of once-a-week training program on physical fitness and metabolic control in children with IDDm. Diabetes Care 12:737–740.
Laaksonen, D.E., M. Atalay, L.K. Niskanen, J. Mustonen, C.K. Sen, T.A. Lakka, and M.I. Uusitupa (2000). Aerobic exercsie and the lipid profile in type 1 diabetic men: a randomized controlled trial. Med. Sci. Exerc. Sports 32:1541–1548.
Landt, K.W., B.N. Campaigne, F.W. James, and M.A. Sperling (1985). Effects of exercise training on insulin sensitivity in adolescents with type I diabetes. Diabetes Care 8:461–465.
Ludvigsson, J. (1980). Physical exercise in relation to degree of metabolic control in juvenile diabetics. Acta Paediatr. Scand. Suppl. 283:45–48.
MacDonald, M.J. (1987). Postexercise late-onset hypoglycemia in insulin-dependent diabetic patients. Diabetes Care 10:584–588.
Mandroukas, K., M. Krotkiewski, G. Holm, G. Stromblad, G. Grimby, H. Lithell, Z. Wroblewski, and P. Bjorntrop (1986). Muscle adaptations and glucose control after physical training in insulin dependent diabetes mellitus. Clin. Physiol. 6:39–52.
Mosher, P.E., M.S. Nash, A.C. Perry, A.R. LaPerriere, and R.B. Goldberg (1998). Aerobic circuit exercise training: Effect on adolescents with well-controlled insulin dependent diabetes mellitus. Arch. Phys. Med. Rehabil. 79:652–657.
Moy, C. S., T. J. Songer, R.E. LaPorte, J.S. Dorman, A.M. Kriska, T.J. Orchard, D.J. Becker, and A.L. Drash (1993). Insulin dependent diabetes mellitus, physical activity and death. Am. J. Epidemiol. 137:74–81.
Nazar, K., J. Taton, J. Chwalbinska-Moneta, and Z. Brzezinska (1975). Adrenergic responses to sustained handgrip in patients with juvenile-onset-type diabetes mellitus. Clin. Sci. Molec. Med. 49:39–44.
Nesher, R., I.E. Karl, and D.M. Kipnis (1985). Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am. J. Physiol. (Cell Physiol.) 249:C226–C232.
Newkumet, K. M., M. M. Goble, R.B. Young, P.B. Kaplowitz, and R.M. Schieken (1994). Altered blood pressure reactivity in adolescent diabetics. Pediatrics 93:616–621.
Nugent, A.-M., I. C. Steele, F. al-Modaris, S. Vallely, A. Moore, N.P. Campbel, P.M. Bell, K.D. Buchanan, E.R. Trimble, and D.P. Nicholls (1997). Exercise responses inpatients with IDDM. Diabetes Care 20:1814–1821.
Pan, X., G. Li, Y.H. Hu, J.X. Wang et al. (1997). Effects of diet and exercsie in the preventing NIDDM in people with impaired glucose tolerance. Diabetes Care 20:537–544.
Peirce, N.S. (1999). Diabetes and exercise. Br. J. Sports Med. 33:161–173.
Perry, T.L., J.I. Mann, N.J. Lewis-Barned, A.W. Duncan, W.A. Waldron, and C. Thompson (1997). Lifestyle intervention in people with insuiln-dependent diabetes mellitus (IDDM). Eur J. Clin. Nutr. 51:757–763.
Ploug, T., H. Galbo, and E.A. Richter (1984). Increased muscle glucose uptake during contractions:no need for insulin. Am. J. Physiol. (Endocrinol. Metab.) 247:E726–E731.
Raguso, C.A., A.R. Coggan, A. Gastaldelli, L.S. Sidossis, E.J. Bastyr III, and R.R. Wolfe (1995). Lipid and carbohydrate metabolism in IDDM during moderate and intense exercise. Diabetes 44:1066–1074.
Rogers, M.A. (1989). Acute effects of exercise on glucose tolerance in non-insulin-dependent diabetes. Med. Sci. Sports Exerc. 21:362–368.
Schiffrin, A., and S. Parikh (1985). Accommodating planned exercise in type 1 diabetic patients on intensive treatment. Diabetes Care 8:337–343.
Skyler, J.S. (1998). Microvascular complications. Retinopathy and nephropathy. Endocrinol. Metab. Clin. North Am. 30:833–856.
Torffvit, O., J. Castenfors, U. Bengtsson, and C.D. Agardh (1987). Exercise stimulation in insulin-dependent diabetics, normal increase in albuminuria with abnormal blood pressure response. Scand. J. Lab. Invest. 47:253–259.
Tuomilehto, J., J. Lindstrom, et al.: Finnish Diabetes Prevention Study Group (2001). Prevention of Type 2 Diabetes Mellitus by changes in lifestyle among subejcts with impaired glucose tolerance. N. Eng. J. Med. 344:1343–1350.
Vinik, A., and T. Erbas (2002). Neuropathy. In: N.B. Ruderman, J.T. Devlin, S.H. Schneider, and A. Kriska (eds.) Handbook of Exercise in Diabetes. Alexandria, VA:American Diabetes Association, pp. 463–496.
Wahren, J. (1979). Glucose turnover during exercise in healthy man and in patients with diabetes mellitus. Diabetes 28:82–88.
Wallberg-Henriksson (1989). Acute exercise:fuel homeostasis and glucose transport in insulin-dependent diabetes mellitus. Med. Sci. Sports Exerc. 21:356–361.
Wallberg-Henriksson, H., R. Gunnarsson, J. Henriksson, J. Ostman, and J. Wahren (1984). Influence of physical training on formation of muscle capillaries in type I diabetes. Diabetes 33:851–857.
Wallberg-Henriksson, H., R. Gunnarsson, S. Rossner, and J. Wahren (1986). Long-term physical training in female type1 (insulindependent) diabetic patients: Absence of significant effect on glycemic control and lipoprotein levels. Diabetologica: 29:53–57.
Wasserman, D.H., S.N. Davis, et al. (2002). Fuel Metabolism during exercise in health and disease. In: N.B. Ruderman, J.T. Devlin, S.H. Schneider, and A. Kriska (eds.) Handbook of Exercise in Diabetes. Alexandria, VA: American Diabetes Association, pp. 66–99.
Waxman, S., and R. Nesto (2002). Cardiovascular Complications. In: N.B. Ruderman, J.T. Devlin, S.H. Schneider, and A. Kriska (eds.) Handbook of Exercise in Diabetes. Alexandria, VA: American Diabetes Association, pp. 415–431.
Zinman, B., S. Zuniga-Guajardo, and D. Kelly (1984). Comparison of the acute and long-term effects of exercise on glucose control in type I diabetes. Diabetes Care 7:515–519.
VOLUME 16 (2003) NUMBER 3 SUPPLEMENT
Sports Science Exchange 90
DIABETES AND EXERCISE: TIPS FOR BETTER PERFORMANCE
With proper experience, planning, conditioning, and strategies for managing diet and insulin, the person with uncomplicated diabetes can engage in any type of exercise at any level of intensity. The goal is to complete the exercise and recovery period with minimal changes in blood glucose. For non-obese people with Type 2 diabetes mellitus (insulin is produced by the pancreas but is ineffective at stimulating glucose uptake from the blood into the cells of the body) who can control their disease simply with diet and regular exercise, no additional precautions are required. The key components of a successful regimen for those with Type 1 diabetes mellitus (no insulin is produced) are to reduce the amount of insulin administered prior to exercise and/or to supplement the diet with carbohydrate. While these are seemingly simple strategies, it is the fine-tuning of these actions that spells success or failure.
Many of the recommendations that follow have been adapted from the publications cited in the list of suggested additional resources at the end of this supplement.
What is the Optimal Time of Day for Exercise?
Disturbances in blood glucose are less likely if exercise is performed in the morning before breakfast and before the morning administration of insulin. This is because circulating insulin is low at this time, and if a regular meal was consumed the night before, both liver and muscle glycogen stores should be filled.
What Should Be Done Before Exercise?
1. Measure blood glucose concentration to determine how well it is under control.
- If blood glucose is <5 mM (90 mg/dl), extra carbohydrate before exercise will likely be required.
- If blood glucose is 5–15 mM (90–270 mg/dl), extra carbohydrate may not be required.
- If blood glucose is >15 mM (270 mg/dl), delay exercise and measure urine ketones.
a. If urine ketones are negative, exercise can be performed, and extra carbohydrate is not required.
b. If urine ketones are positive, take insulin and delay exercise until ketones are negative.
2. Determine the appropriate pre-exercise carbohydrate meal.
Before exercise one can estimate the intensity, duration, and the energy requirement of the exercise by consulting standard tables. By dividing the estimated calorie requirement by four (each gram of carbohydrate is equivalent to four calories), the potential carbohydrate requirement in grams can be predicted. Diabetics should eat or drink an appropriate carbohydrate-containing snack or meal 1–3 h prior to exercise. This food or beverage should contain about 15 g of carbohydrate per 30 min of anticipated moderate-intensity exercise. Foods such as fig bars, crackers, yogurt, muffins, oatmeal cookies, soups, dried fruit, bread sticks, and granola bars are appropriate. Drinks that contain simple carbohydrates and electrolytes are excellent for helping avoid hypoglycemia and plasma volume depletion during exercise (for example, an 8-oz serving of Gatorade contains 14 G of carbohydrate). Even whole milk, skim milk, and orange juice are better than water alone On the other hand, meal replacement drinks designed to provide complete supplementation, i.e., carbohydrate, fat and protein, can lead to an inappropriate rise in blood glucose during and after exercise.
3. Administer the appropriate pre-exercise insulin dose.
- Inject insulin (or adjust the output of an insulin pump) about 1 hour before exercise.
- Decrease the dose of insulin so that the greatest increase in circulating insulin does not occurs during the exercise period.
- Do not use an arm or leg that will be involved in exercise as an injection site and be sure that the insulin is injected into subcutaneous tissue not muscle.
What Should Be Done During Exercise?
1. Monitor blood glucose during long exercise sessions. For running, cycling, swimming and other endurance types of activities, this may require setting a circular course so that glucose meters are periodically available.
2. Always replace fluid losses adequately. The goal should be to replace all or nearly all of the body weight lost as sweat during the exercise period itself. This weight loss can be estimated by recording the difference in body weight before and after exercise on prior occasions.
3. If required, use supplemental carbohydrate feedings (an additional 40–50 g for adults, 20–30 g for children) every 60 min during extended periods of moderate intensity exercise. For example, Gatorade restores blood glucose very rapidly during exercise in people with Type 1 DM who are becoming hypoglycemic. Other sports drinks with a similar composition (~6% carbohydrate plus electrolytes) may also be effective but have not been studied.
What Should Be Done After Exercise?
1. Monitor blood glucose, including overnight monitoring if exercise is not habitual and/or is performed in the late afternoon. Avoid alcohol consumption after exercise because alcohol diminishes the ability to monitor marked or subtle feelings that would otherwise alert the person with diabetes to the fact that blood glucose is either too high or too low.
2. Adjust insulin administration downward to decrease immediate and delayed actions of insulin. If required, increase carbohydrate intake for up to 24 hours after activity, depending on the intensity and duration of exercise (more intense and prolonged exercise requires more carbohydrate) and the risk—based on prior experience— of the occurrence of low blood glucose. Ingestion of ~1.5 g carbohydrate/kg body weigh (0.7 g/lb) soon after exercise will help restore muscle and liver glycogen after very prolonged or exhausting exercise. It should be noted, however, that although low blood glucose can occasionally occur several hours after exercise in diabetics, some insulin is needed late after exercise to fully restore muscle glycogen levels.
3. Ingest the appropriate amount of carbohydrate on a daily basis.
The type of exercise—endurance, sprint, resistance, intensity of exercise—high, medium, low, and duration of exercise—brief, moderate, prolonged—(or as in most sports some combination of these) must be considered:
- If aerobic exercise of a moderate intensity is to be undertaken on a daily basis and usually lasts less than 1 hour, the diabetic athlete should ingest 5–6 g of carbohydrate/kg body weight (2.3–2.7 g/lb) on a daily basis.
- If the athlete trains more than 1–2 hours per day, 6–8 g of carbohydrate/kg body weight (2.7–3.6 g/lb) may be required daily.
Which is Worse, Low Blood Glucose (Hypoglycemia) or High Blood Glucose (Hyperglycemia)?
The answer is that both hypoglycemia and hyperglycemia should be avoided whenever possible. For athletic competitions, hypoglycemia must be avoided because fatigue, loss of mental focus, and reductions in strength are obviously not compatible with athletic success. Thus, it may seem reasonable that maintaining a state of hyperglycemia is one way to insure athletic success. In the short run this may work, but the consistent state of hyperglycemia must be avoided because even mild but consistent hyperglycemia significantly increases the likelihood of serious medical complications of diabetes. Unfortunately, some diabetic athletes apparently sacrifice glucose control in favor of avoiding hypoglycemia so they can perform at high levels.
Other Practical Considerations
Here are some additional tips for the diabetic exerciser:
- Frequent glucose monitoring is obviously essential for safe exercise.
- Carry some form of carbohydrate snack (simple sugars).
- Carry medical identification.
- If convenient, exercise with a friend who knows you have diabetes. Carry a cell phone in case of a diabetic emergency.
- Invest in good footgear if walking, jogging, and/or running are among your chosen activities.
- Use extra care to avoid large fluctuations in plasma glucose when exercising in the cold or heat.
SUGGESTED ADDITIONAL RESOURCES
American Diabetes Association (2002). Clinical Practice Recommendations: 2002. Diabetes Care 25 (suppl. 1):S64–S68.
Peirce, N.S. (1999). Diabetes and exercise. Br. J. Sports Med. 33:161–173.
N.B. Ruderman, J.T. Devlin, S.H. Schneider, and A. Kriska (eds.) Handbook of Exercise in Diabetes. Alexandria, VA: American Diabetes Association.
For additional information: In the U.S.A. and Canada: 1-800-616-GSSI (4774)
www.gssiweb.com
This article may be reproduced for non-profit, educational purposes only.