KEY POINTS
- The management of fluctuating blood glucose levels in athletes with type 1 diabetes (T1D) is crucial for both safety and performance during training, sport and competition. Low blood glucose (hypoglycemia) is a major barrier to most forms of exercise, but activity-related high blood glucose levels (hyperglycemia) can also occur with some forms of intense exercise and when insulin dose adjustments are suboptimal for exercise.
- Continuous glucose monitors (CGM) offer real-time insights into interstitial glucose levels, as a proxy for circulating blood glucose concentrations, for these individuals and their coaching and support teams.
- Endurance and resistance training present unique challenges in glucose self-management for physically active individuals with T1D, as aerobic exercise generally decreases glucose levels while anaerobic exercise keeps glucose more stable or can increase it. With competition, glucose levels may rise because of stress hormones, but then glucose levels can drop into the hypoglycemic range (low blood glucose levels) in recovery. Proactive blood glucose measures guided by CGM are critical.
- CGM data helps to inform carbohydrate intake strategies for training and competition, and to help guide more appropriate insulin adjustments for different forms of activity (e.g., aerobic, anaerobic, mixed), with the primary goal to reduce the occurrence of both hypo- and hyperglycemia. Post-exercise CGM monitoring is crucial, with the impact on glycemia of a single bout of exercise lasting for at least 24 hr in recovery.
- Time in Range (TIR) metrics from CGM data across a longer period, generally from 24 hr to 3 months, can help guide clinical decision-making by health care providers for the athletes training and competition. Primary goals include a minimum percent time in the so called “safe” or “target” glucose range of 70-180 mg/dL (3.9-10.0 mmol/L) for ≥ 70% of time, with minimal exposure to glucose < 70 mg/dL (< 3.9 mmol/L) for < 3.0% of the time.
INTRODUCTION
Individuals living with type 1 diabetes (T1D) face the unique challenge of constantly managing fluctuating blood sugar (glucose) levels, a task that can become even more difficult during sport and exercise activities. For the athlete with T1D, good glucose management is crucial for both performance and safety, but the capacity to demonstrate “normal” glucose responses to exercise and sport is limited by the non-physiologic delivery of insulin and disruption in endogenous glucagon responses to exercise (Riddell et al., 2020). While athletes without diabetes typically have glucose within a relatively tight range in sport and competition (between 70-120 mg/dL [3.9-10.0 mmol/L]) (Skroce et al., 2024), athletes with T1D develop hypo- (<70 mg/dL [<3.9 mmol/L]) and/ or hyperglycemia (>180 mg/dL [>10.0 mmol/L]) before and following activity, even when insulin therapy is intensified, and glucose levels are monitored (Weenen et al., 2023).
Despite the additional challenges faced during training and competition, the visibility of elite-level athletes with T1D have gained attention on a global scale. These athletes include entire professional sports teams, such as the Novo Nordisk cycling team, which is comprised solely of individuals with T1D, and ten-time US Olympic swim medallist Gary Hall Jr. Additionally, there are athletes who serve as active spokespersons for diabetes technology companies, such as National Hockey League (NHL) player and continuous glucose monitor (CGM) user Max Domi. This increased visibility and/or prevalence in athletes with T1D in sports may be due, in part, to the progression of diabetes technology in the past decade. In particular, the advent of CGM which provide real-time insights into circulating (interstitial) glucose levels and trends, have revolutionized glucose management for individuals living with T1D. The aim of this Sports Science Exchange (SSE) article is to provide athletes with T1D and their coaching team with a guide on how to harness CGM technology to obtain athletic goals while effectively managing T1D.
TYPE 1 DIABETES
Type 1 diabetes is a form of diabetes mellitus resulting from the selective autoimmune destruction of the pancreatic β cells and the complete or near complete absence of insulin production. This form of diabetes, previously called “juvenile diabetes” or “insulin-dependent diabetes” accounts for 5-10% of all cases of diabetes, depending on the geographical region (Liu et al., 2020). While a diagnosis of T1D can come at any age, the highest incidence rate of diagnosis occurs in the teenage years (Rogers et al., 2017). Although distinguishing diabetes type can be challenging when overweight, obese or older individuals present with hyperglycemia (glucose > 180 mg/dL [> 10.0 mmol/L]), confirmation of a T1D diagnosis can be performed by measuring T1D-specific autoimmune biomarkers and/or c-peptide levels (Sacks et al., 2023). These biomarkers reflect the autoimmune dysfunction within the pancreatic beta-cells, along with the resultant loss in insulin production to zero (or near zero), which is exclusively associated with this form of diabetes. If left untreated with daily insulin therapy, these individuals rapidly develop severe hyperglycemia (glucose > 350 mg/dL [> 19.4 mmol/L]) and life-threatening diabetic ketoacidosis.
Clinical Management and Goals
The clinical management of T1D involves daily insulin therapy, which aims to enhance the individual's quality of life by preventing and alleviating the disease's acute and chronic complications. Given the strong relationship between diabetes-related complications (both micro- and macrovascular disease) and prolonged hyperglycemia exposure (Gubitosi-Klug & Group, 2014), modern management of T1D focuses on glucose monitoring with intensive insulin therapy. This typically includes basal/bolus insulin delivery to help manage glucose levels overnight, in between meals and after meals. Insulin therapy could involve either multiple daily injections (MDI) of basal/bolus insulins (i.e., long-acting and short-acting insulins for between meals and mealtime, respectively) or the use of a continuous subcutaneous insulin infusion (CSII) device, commonly known as an insulin pump. In recent years, automated insulin-delivery (AID) systems, integrating an algorithm-facilitated insulin pump and CGM to help guide insulin delivery requirements, have been more widely adopted for better glucose management in T1D. These AID systems still require manual insulin boluses at mealtimes, and ideally carbohydrate “counting” to better estimate insulin needs with nutrition, but have been shown to increase the overall time that glucose levels are within the target range, particularly in those who were far from reaching clinical targets for glucose control (Crabtree et al., 2023). At present, AID systems have been adopted by ~20% of the North American T1D population (Miller et al., 2020), while CGM and standard pump use is higher (~66% of youth and young adults with T1D use CGM regularly), but with considerable regional differences in overall diabetes-related technology adoption, likely because of various access barriers, costs and insurance coverage (Prahalad et al., 2024).
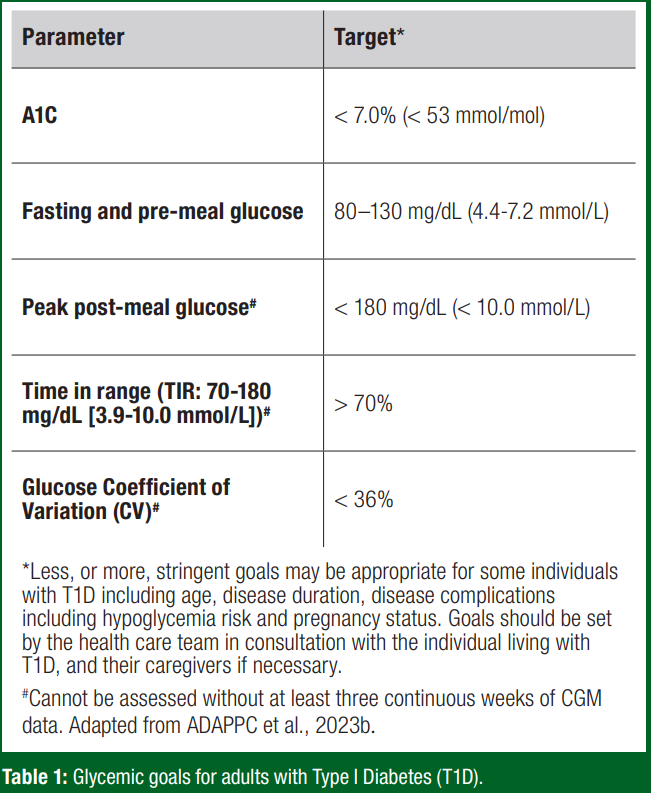
Glycemic management goals in diabetes, including individuals with T1D, are contingent upon the individual's age and pregnancy status, with various glucose metrics considered when evaluating overall glucose “control” in T1D (Table 1). These glycemic targets can be assessed through frequent whole blood glucose monitoring (i.e., 5-7 times daily), regular glycosylated hemoglobin A1C blood tests and, with regular usage, CGM analytics programs provide substantially more data interpretation (time in range (TIR), glycemic variability, etc).
Physical Activity and T1D
Regular physical activity and planned exercise training sessions are crucial components of managing T1D. However, managing glucose levels with exercise and sport is extremely difficult, even for the experienced athlete with this disorder (Colberg et al., 2021; Lespagnol et al., 2020; Moser et al., 2020a, b; Ratjen et al., 2015; Scott et al., 2020). Nonetheless, regular exercise has numerous health benefits for the individual living with T1D, including the enhancement of insulin sensitivity, more time in glucose target range and reductions in various cardiovascular disease risk factors. For instance, a 45-min walk can lower glucose levels in adults with T1D if insulin is not reduced enough, or if carbohydrates are not consumed to offset the activity-induced increase in insulin sensitivity and enhancement in glucose disposal rate from contraction-mediated glucose uptake (Rickels et al., 2018).
In individuals with T1D, the risk of activity-induced hypoglycemia is around 30-40% if insulin dose adjustments are not made or if carbohydrate feeding does not occur (Riddell et al., 2019). Endurance athletes with T1D also need to adjust their insulin regimens daily and consume high amounts of simple carbohydrates to help maintain optimal glycemia during training and competition (Pitt et al., 2022; Scott et al., 2019). Some reduce bolus insulin doses by over 50% during a 5-day cycling race, while others consume high amounts of carbohydrates (> 60 g/ hr) without taking any bolus insulin (Moser et al., 2020a). Recreational adults with T1D also experience significant reductions in blood glucose levels during exercise and thus require significant reductions in basal and bolus insulin during training and competition (Moser et al., 2020b). Both competitive and non-competitive athletes with T1D frequently experience considerable time outside their glucose target range for competition (70-180 mg/dL [3.9-10.0 mmol/L]) (Lespagnol et al., 2020; Pitt et al., 2022; Scott et al., 2020; Weenen et al., 2023). While most endurance activities lower blood sugar levels in individuals with T1D, brief and intense exercises can cause dramatic increases in glucose concentration (Riddell & Peters, 2023). The American Olympian Gary Hall Jr had a large rise in his glucose level from ~140 mg/dL (7.8 mmol/L) to over 300 mg/dL (16.7 mmol/L) in a single freestyle swim that lasted under 22 s at the Sydney Olympics, simply because of the rise in his adrenaline associated with the competition (personal communication to MC Riddell).
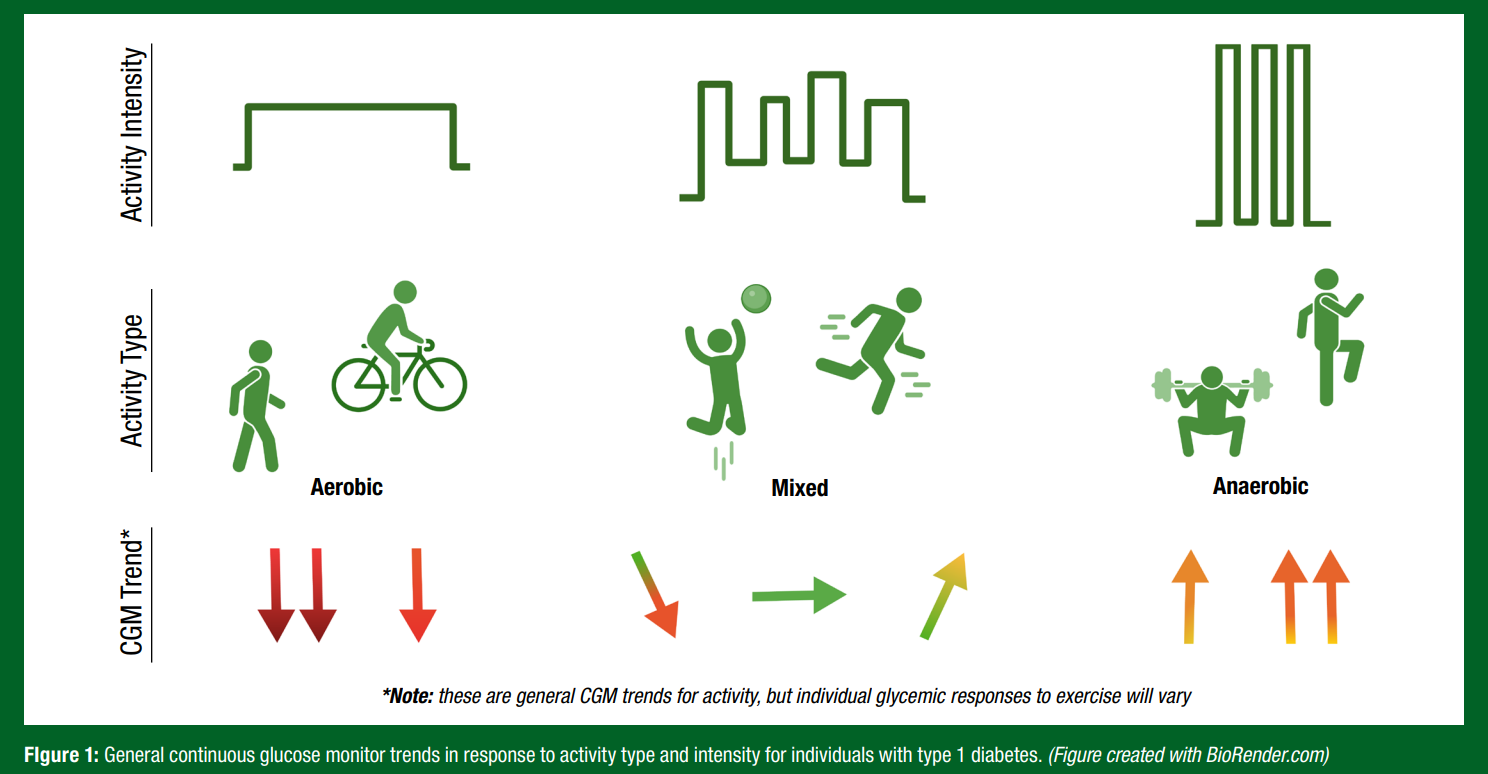
Exercise Type and Glucose Response in T1D
Each athlete with T1D will have a differing response to any given activity, which can even vary within an individual on a day-to-day basis depending on factors including time of day, insulin delivery, hydration status, carbohydrate consumption and competition stress (Riddell et al., 2017). In general, as outlined in Figure 1, individuals with T1D will face drops in glucose with aerobic activity, rises in glucose with anaerobic activity and mitigating effects if the activity is mixed in nature (Riddell et al., 2017).
CONTINUOUS GLUCOSE MONITORING IN T1D AND EXERCISE
Due to the rapid, and sometimes unpredictable, changes in glucose levels during activity, the use of CGM is instrumental in the self-management of the athlete with T1D (Riddell et al., 2020). It is hoped that the use of CGM by the athlete with T1D allows for ongoing therapy evaluation and for more proactive measures to limit glucose fluctuations during training, competition and recovery (Moser et al., 2020c). The following sections describe how a CGM works, target glucose ranges for T1D athletes in the context of competition, nutritional considerations for the athlete and considerations for the athlete and practitioner (coaches, support team, etc.) when using a CGM.
What is a CGM and How Does it Work?
A CGM is first and foremost considered “wearable technology” allowing the user to view their glucose concentrations continuously and in near real time. However, for the individual living with T1D it is also considered a part of their “standard of care” for their diabetes management, since the CGM can help inform insulin dosing and perhaps even inform the individuals AID system, if they choose to be wearing one.
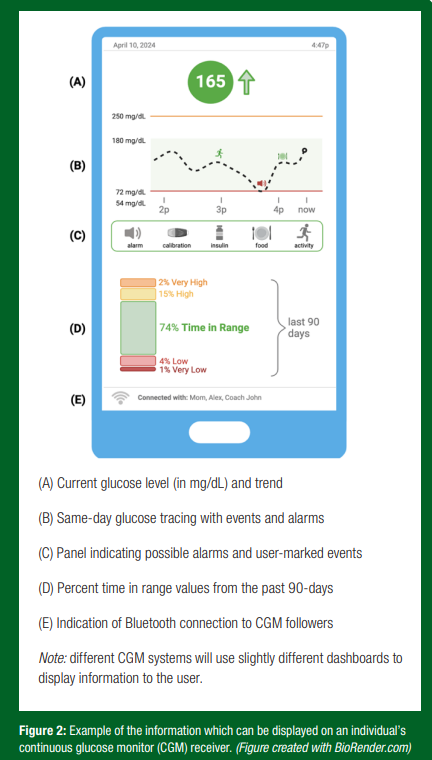
While many readers may be familiar with the more classic “fingerstick” blood glucose meter, which provides a snapshot of blood glucose concentration at the time of testing, a CGM serves a similar purpose but with enhanced glucose data acquisition rates and analytics. By continuously measuring a user’s glucose level in the subcutaneous fluid just under the skin, termed interstitial/sensor glucose, a CGM device provides information equivalent to ~300 or more fingerstick measurements per day. This type of glucose monitoring offers the T1D athlete an idea of the in-between moments and glucose trends, which may otherwise be overlooked by a fingerstick glucose reading. While multiple CGM systems are commercially available, they all use three main components to provide users with glucose readings and additional information in ~5-min increments (Figure 2):
- Sensor: A flexible, thin microwire that lies under the skin to measure glucose in the interstitial space - the area between the skeletal muscle blood vessels and cells. The sensor is inserted using an automatic insertion device and secured with adhesive. Sensors are disposable and last from 10-14 days.
- Transmitter: A Bluetooth or radio-frequency component, roughly the size of a dime, that is attached to the sensor filament and housing adhesive that sits on top of the skin. Transmitters may be rechargeable or disposable, with sensor life generally lasting 7-14 days. *Note, some newer CGM systems integrate the sensor and transmitter into one disposable component that lasts about 10-14 days.
- Receiver: A stand-alone device containing proprietary software that reports the sensor glucose level. The software can be installed on the user’s smartphone which can display glucose on a secondary device, such as a smartwatch. The display (Figure 2) may show several real-time and historic glucose trend data such as:
a. Current glucose readings (i.e. 200 mg/dL [11.1 mmol/L]).
b. Current glucose trend arrow (i.e. rising, falling, steady).
c. Alerts and alarms: Replace sensor, out-of-range, rapid glucose rise or fall, high glucose, low glucose, urgent low glucose.
d. Past glucose trends: A line graph of the last 24-hours. Some systems can also show glucose time in range, patterns and trends for up to the last 3 months.
e. User-marked events: Including food intake, insulin delivery, activity, finger-stick calibration.
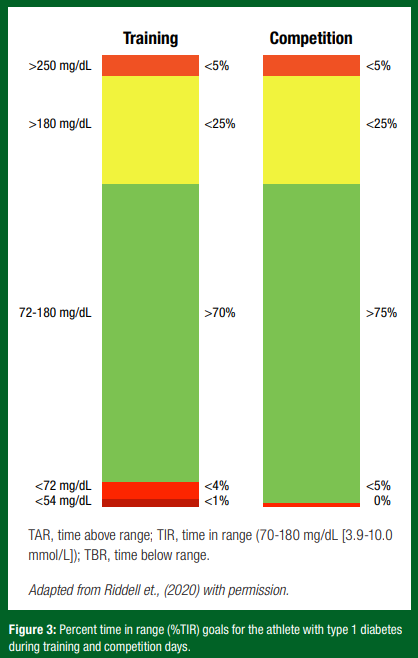
CGM Targets for the Athlete with T1D
What is time in range? Some CGM platforms will offer the user reports on time in range (defined as percent time between 70-180 mg/dL [3.9-10.0 mmol/L] for people living with diabetes). This, and the other associated metrics, may be displayed over any given period (i.e., day, week, month) that an individual’s glucose reading is either low (time below range; TBR), in target range (time in range; TIR) or above the target range (time above range; TAR). To inform actionable training decisions, these percent values can be converted into time durations (hrs, mins). For instance, if an athlete goes on a 1-hr training run and has 10% TAR, that means they spent 6 min of that run with high blood glucose.
How could time in range targets differ on training and competition days? The guidelines for time in range on a training day are in line with general recommendations for health in T1D (Riddell et al., 2020). However, the competition day guidelines are more stringent (Figure 3). The focus shifts to an increased TIR and limited TBR. This is an important adjustment as low glucose can impact performance and may even lead to not completing a competition event (Riddell et al., 2020).
How can the visibility of CGM trends inform training and competition? As discussed above, different forms of exercise can have varying effects on an athlete’s glucose. One of the most significant advantages of using a CGM for athletes with T1D - along with their coaches, teammates and family members, where applicable - is the ability to observe real-time interstitial glucose levels and trends. This visibility enables athletes to make proactive decisions regarding high or low glucose levels, which may otherwise impair their performance and possibly safety as acute changes in glucose are linked to mild cognitive dysfunction (Cox et al., 2005). Moreover, since CGM systems store data on various platforms, individuals can retrospectively examine their patterns related to different types of training, durations, timings, intensities, days and even competition stress. This could help determine the effectiveness of their performance strategies. While dedication and support are crucial, using CGM may help the athlete with T1D achieve the recommended glucose targets and enhance performance.
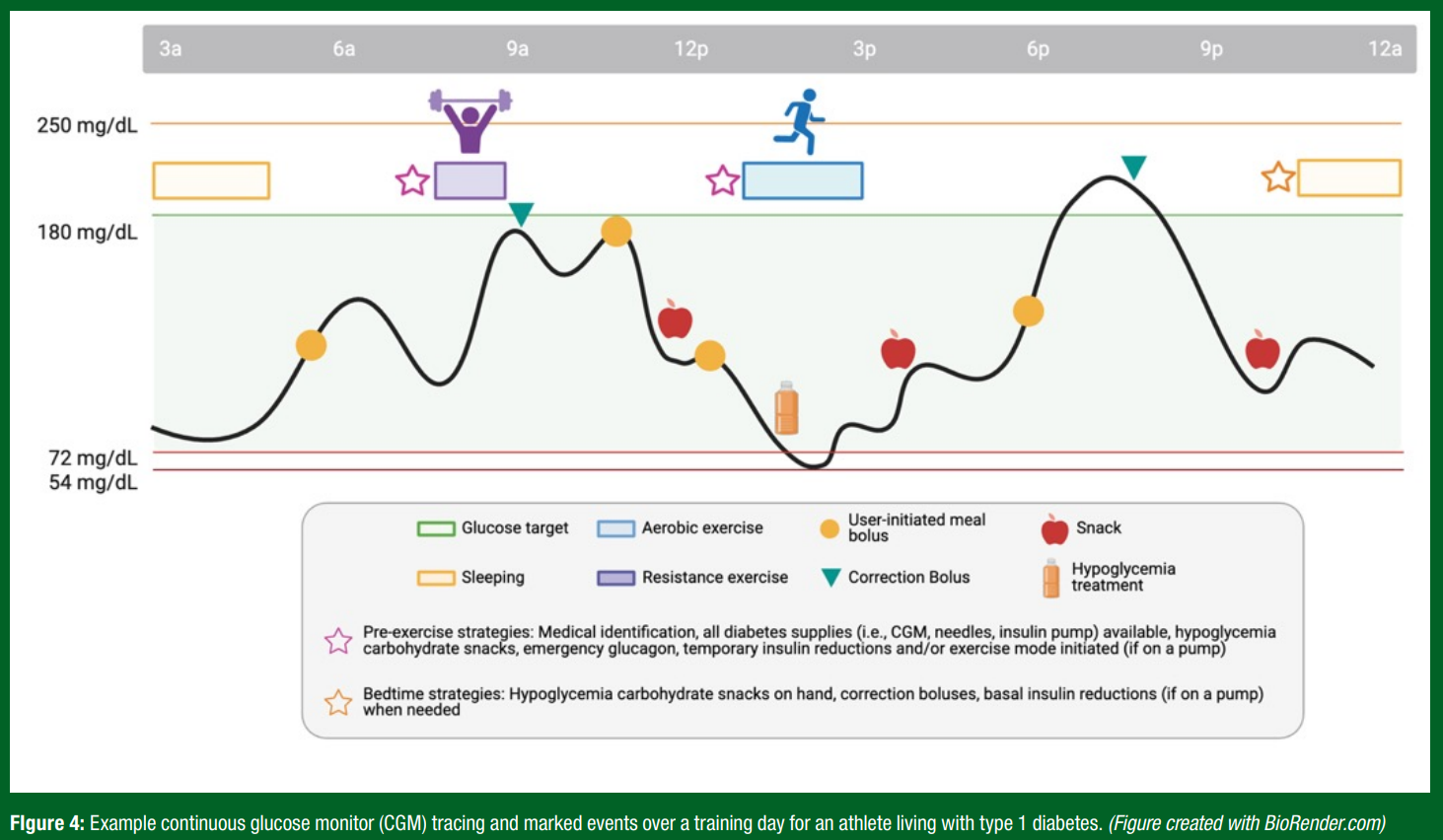
Using CGM for fuelling during sport and exercise. While CGMs offer glucose trends, tracings, alerts and reports (Figure 4), a CGM will not offer treatment solutions. It is the athlete with T1D, their coaching staff and support team who will ultimately make treatment decisions and Figure 4 outlines how CGM tracings and trends can inform treatment decisions for an athlete with T1D.
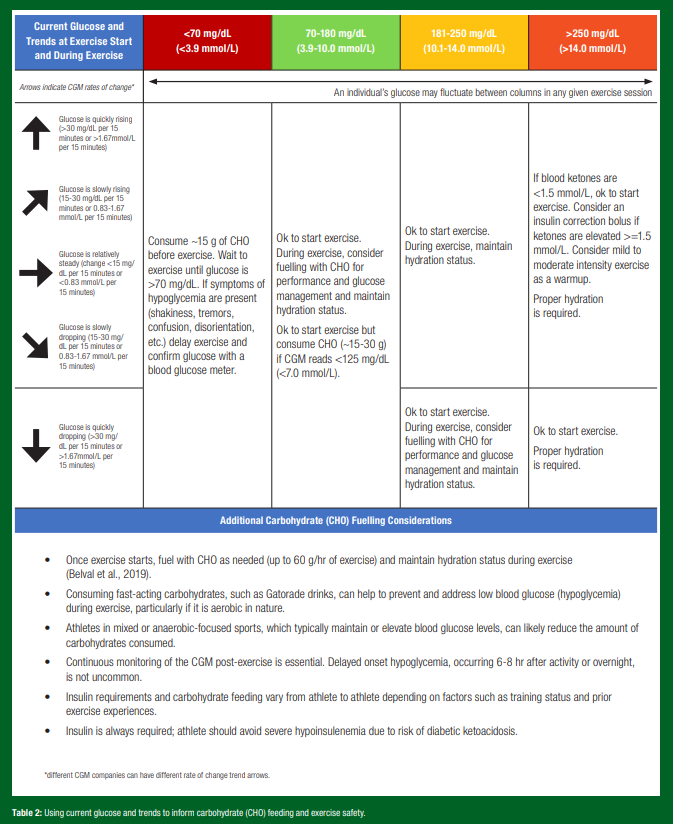
CGM trends to inform fuelling. Based on a recent CGM exercise consensus document (Moser et al., 2020c), Table 2 offers some guidance on how CGM can inform fuelling during activity.
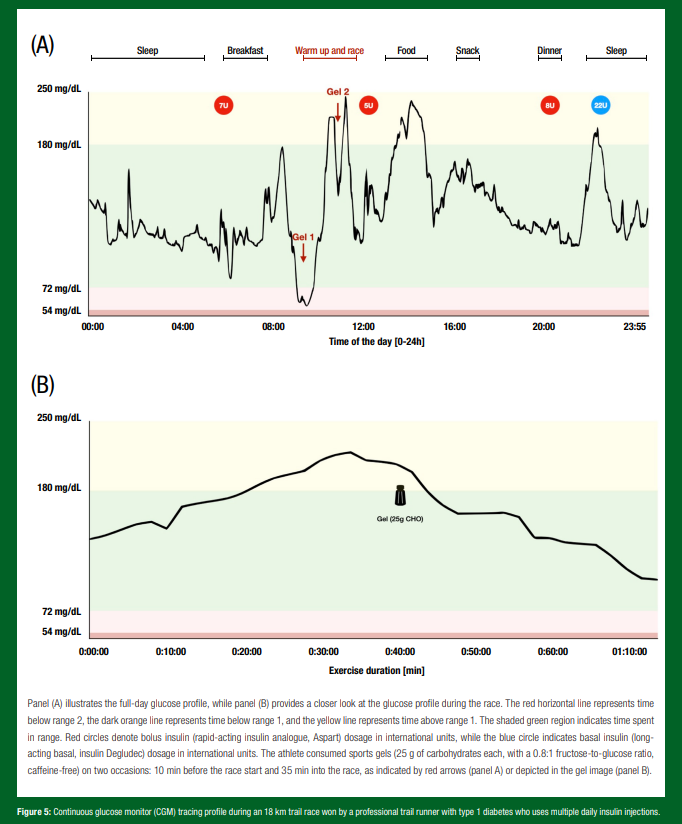
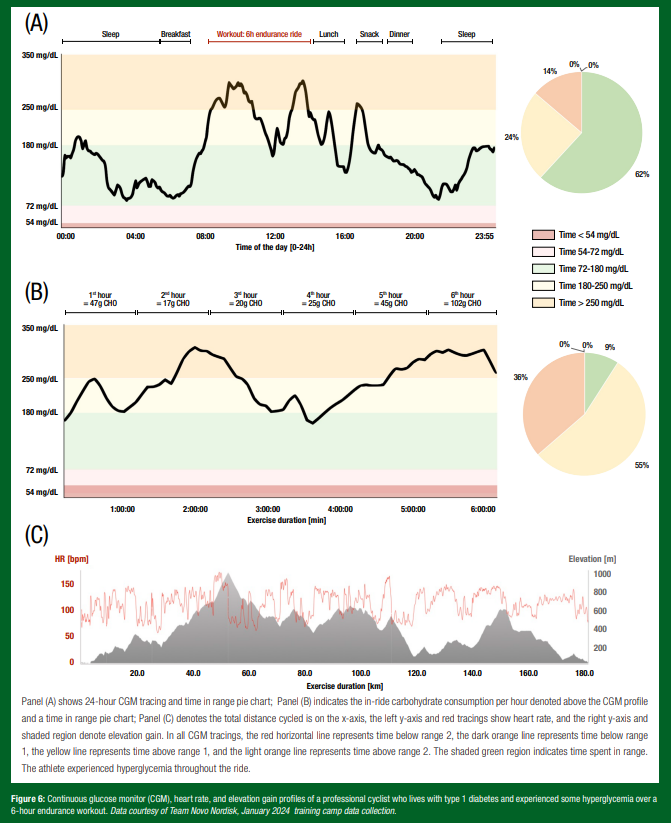
Nutritional requirements for the athlete with T1D. Nutritional recommendations for athletes with T1D should be individualized, considering factors like weight goals, energy requirements, type of exercise routine, environmental factors and overall glycemia as measured by CGM or self-monitoring of blood glucose levels. Figures 5 and 6 depict real-world tracings from athletes living with T1D, shedding light on the individuality of nutrition and treatment decisions for athletes managing T1D.
The American Diabetes Association Standards of Care now emphasize foods, food selections and dietary patterns (ADAPPC, 2023a). They underscore that nutrition therapy is important in the overall management of diabetes and advocate for individualized eating plans. Athletes with T1D do not appear to have different nutritional requirements from non-diabetic athletes, but they often need to modulate their carbohydrate (CHO) intake based on their glucose levels, and they need to change their insulin management plan (i.e., amount of insulin administered) accordingly for different meals and for exercise (Cavallo et al., 2024; Gallen et al., 2011; Riddell et al., 2020). Additionally, a pre-race taper period (i.e., maintenance of high intensity and lower training volume) can interfere with daily glucose trends as well as insulin requirements. Individual, day-by-day approaches can temper glucose oscillations. Post exercise recovery also requires a modification to insulin therapy (typically a reduction in insulin delivery), along with appropriate re-fuelling with carbohydrates and protein, and vigilant glucose monitoring to help guard against post-exercise dysglycemia (Scott et al., 2021).
Flexibility in food choices and meal timings is crucial given the profound impact of food intake on min-by-min glycemia for individuals with T1D, which can be highlighted using a CGM. For instance, an endurance athlete with T1D may require over 90 g/hr of simple CHO, along with fluids, to sustain glycemia and endurance performance (Pitt et al., 2022). Current guidelines suggest consuming 5-12 g CHO/kg body mass/day (depending on training load) in athletes with T1D (Gallen et al., 2011). Conversely, some individuals may opt to restrict CHO intake to mitigate total daily insulin requirements and minimize post-meal hyperglycemia or hypoglycemia (CDC, 1987; Schmidt et al., 2019). Protein consumption might need to be higher during training sessions than during competitions to support optimal muscle maintenance and repair (Pitt et al., 2022). Many individuals with T1D may aim for weight loss, thus attempting to restrict total caloric intake while mitigating the risk of insulin or activity-induced hypoglycemia (Colberg et al., 2021). In summary, there's no one-size-fits-all approach to nutrition therapy, however, the use of CGM can highlight glycemic responses in everyday situations and throughout training and competition may help inform the correct approach for the athlete with T1D.
OTHER CGM CONSIDERATIONS
Sensor Accuracy and Lag Time
CGM accuracy, determined by how close an interstitial glucose value is to a true blood glucose sample taken at the same time, is relatively good for all CGM systems (within a 15% difference). However, it is crucial for athletes and their support systems to understand that CGMs measure interstitial glucose values, which can potentially lag behind changes in blood glucose by ~10-20 min (Wadwa et al., 2018; Zaharieva et al., 2019), especially during rapid glucose changes during exercise (Jin et al., 2023; Zaharieva et al., 2019). Therefore, if athletes experience symptoms of high or low glucose, they should confirm using a fingerstick, and calibrate the CGM to this reading if they differ. To stay proactive, athletes could also consider adding fingerstick calibrations during specific times their sport allows, such as during a bench period in soccer or basketball.
Another common period of inaccuracy occurs when a sensor experiences undue pressure resulting in falsely low glucose readings, termed "compression lows”, or lost connections (Mensh et al., 2013). This can occur with tight compression clothes worn with some sports (i.e., cycling shirt) or most often, it is seen during sleep when an individual lies on their sensor. If athletes with T1D exhibit patterns of overnight high or low glucose, proactively setting additional alarms to confirm glucose readings during this period may prove beneficial. Lastly, some CGM readings are influenced by external factors like hydration status, heat or medications (e.g., aspirin, Tylenol). It's essential to consult specific CGM manufacturer guidelines for further information on managing these influences.
Wearability
- Sensor placement: Sensor placement locations, such as the back of the upper arm, abdomen, lower back and/or buttocks, may vary slightly among CGM systems. Ensure the sensor is positioned at least one inch away from insulin injection sites (for MDI users) or infusion sites (for pump users). While rotating sites with each sensor insertion is important, athletes with T1D may also need to consider their sport-specific requirements. For instance, an individual who plays a contact sport may need to consider an area where the CGM will be less likely to be hit or pulled off, while a gymnast may wish to avoid an area like the lower back where rolling on the sensor could occur.
- Skin reactions: Some individuals with T1D experience skin reactions to the CGM adhesive, which could be intensified by sweat and heat - conditions commonly experienced by athletes. Cleansing the skin with alcohol wipes or soap and water, letting the skin dry completely before insertion and/or using a skin barrier (such as a liquid barrier wipe, spray or tape with a hole cut in the centre for sensor insertion) may mitigate skin reactions.
- Keeping the sensor on: Sweat, heat, water and contact are all conditions faced by athletes with T1D which can cause the CGM adhesive to wear down and the CGM to fall off before the end of the wear period. Using a liquid adhesive before putting on the sensor or an overlay patch or medical tape over the sensor following insertion may help.
- Water sports: All CGMs are water-resistant, but to avoid failure, individuals should consult manufacturer specific guidelines on water submersion depth and time. Water submersion can reduce Bluetooth connectivity, so athletes participating in water sports may need to ensure the sensor and receiver are within a closer range during training and/or competition.
CONCLUSION
Continuous glucose monitoring is one of the most beneficial tools for the management of T1D during sports and exercise. By providing real-time insights into glucose levels and trends, CGM can inform proactive adjustments in insulin dosing, carbohydrate feeding and even training decisions to improve diabetes management for the athlete with T1D. Integrating CGM data into decision-making for individuals with T1D and their support teams may enhance the experiences and accomplishments of athletes with T1D.
DISCLOSURES
Over the last 12 months, MCR has served on scientific advisor boards for embecta, Dexcom Inc, Eli Lilly, Indigo Diabetes, Insulet, Novo Nordisk, Spiden AG, Supersapiens, Zealand Pharma and Zucara Therapeutics. MCR has also provided Continuing Medical Education lectures for Dexcom, Novo Nordisk, Sanofi, Roche Diabetes Care and Insulet. MCR’s research has been supported with funding (or in-kind contributions) from Dexcom, Eli Lilly, Supersapiens and Zucara Theraputics. MCR hold stock and/or commercial interest in Supersapiens and Zucara Therapeutics. KS was a consultant for Supersapiens (TT1 Products, INNC, Atlanta, GA, USA) until 1st of March 2024. Supersapiens provided sensors, but no funding for KS's research. JMH is employed by the Gatorade Sports Science Institute, a division of PepsiCo R&D. The views expressed are those of the authors and do not necessarily reflect the position of policy of PepsiCo, Inc. All other authors have no disclosures to declare.
REFERENCES
American Diabetes Association Professional Practice Committee (ADAPPC) (2023a). N.A. ElSayed, G. Aleppo, R.R. Bannuru, E.A, Beverly, D. Bruemmer, B.S. Collins, A. Darville, L. Ekhlaspour, M. Hassanein, M.E. Hilliard, E.L. Johnson, K. Khunti, I. Lingvay, G. Matfin, R.G. McCoy, M.L. Perry, S.J. Pilla, S. Polsky, P. Prahalad, R.E. Pratley, A.R. Segal, J.J. Seley, R.C. Stanton, and R.A. Gabbay. 5. Facilitating positive health behaviors and well-being to improve health outcomes: Standards of care in diabetes—2024. Diab. Care 47(S1):S77–S110.
American Diabetes Association Professional Practice Committee (ADAPPC) (2023b) N.A. ElSayed, G. Aleppo, R.R. Bannuru, E.A. Beverly, D. Bruemmer, B.S. Collins, A. Darville, L. Ekhlaspour, M. Hassanein, M.E. Hilliard, E.L. Johnson, K. Khunti, I. Lingvay, G. Matfin, R.G. McCoy, M.L. Perry, S.J. Pilla, S. Polsky, P. Prahalad, R.E. Pratley, A.R. Segal, J.J. Seley, R.C. Stanton, and R.A. Gabbay. 6. Glycemic goals and hypoglycemia: standards of care in diabetes—2024. Diab. Care 47(S1):S111–S125.
Belval, L.N., Y. Hosokawa, D.J. Casa, W.M. Adams, L.E. Armstrong, L.B. Baker, L. Burke, S. Cheuvront, G. Chiampas, J. González-Alonso, R.A. Huggins, S.A. Kavouras, E.C. Lee, B.P. McDermott, K. Miller, Z. Schlader, S. Sims, R.L. Stearns, C. Troyanos, and J. Wingo (2019). Practical hydration solutions for sports. Nutrients 11:1550.
Cavallo, M., M.D. Fano, L. Barana, I. Dozzani, E. Bianchini, M. Pellegrino, L. Cisternino, S. Migliarelli, C. Giulietti, R. Pippi, and C.G. Fanelli (2024). Nutritional management of athletes with type 1 diabetes: A narrative review. Nutrients 16:907.
Centers for Disease Control and Prevention (CDC) (1987). Update: human immunodeficiency virus infections in health-care workers exposed to blood of infected patients. Morbid. Mortal. Weekly Rep. 36:285–289.
Colberg, S.R., J. Kannane, and N. Diawara (2021). Physical activity, dietary patterns, and glycemic management in active individuals with type 1 diabetes: An online survey. Int. J. Environ. Res. Public Health 18:9332.
Cox, D.J., B.P. Kovatchev, L.A. Gonder-Frederick, K.H. Summers, A. McCall, K.J. Grimm, and W.L. Clarke (2005). Relationships between hyperglycemia and cognitive performance among adults with type 1 and type 2 diabetes. Diab, Care 28:71–77.
Crabtree, T.S.J., T.P. Griffin, Y.W. Yap, P. Narendran, G. Gallen, N. Furlong, I. Cranston, A. Chakera, C. Philbey, M.A. Karamat, S. Saraf, S. Kamaruddin, E. Gurnell, A. Chapman, S. Hussain, J. Elliott, L. Leelarathna, R.E.J. Ryder, P. Hammond, A. Lumb, P. Choudhary, and E.G. Wilmot (2023). Hybrid closed-loop therapy in adults with type 1 diabetes and above-target HbA1c: A real-world observational study. Diab. Care 46:1831–1838.
Gallen, I.W., C. Hume, and A. Lumb (2011). Fuelling the athlete with type 1 diabetes. Diab. Obes. Metab. 13:130–136.
Gubitosi-Klug, R.A. and D.R. Group (2014). The diabetes control and complications trial/ epidemiology of diabetes interventions and complications study at 30 years: Summary and future directions. Diab. Care 37:44–49.
Jin, Z., A.E. Thackray, J.A. King, K. Deighton, M.J. Davies, and D.J. Stensel (2023). Analytical performance of the factory-calibrated flash glucose monitoring system freestyle Libre2TM in healthy women. Sensors 23:7417.
Lespagnol, E., O. Bocock, J. Heyman, F.-X. Gamelin, S. Berthoin, B. Pereira, J. Boissière, M. Duclos, and E. Heyman (2020). In amateur athletes with type 1 diabetes, a 9-day period of cycling at moderate-to-vigorous intensity unexpectedly increased the time spent in hyperglycemia, which was associated with impairment in heart rate variability. Diab. Care 43:2564–2573.
Liu, J., Z.-H. Ren, H. Qiang, J. Wu, M. Shen, L. Zhang, and J. Lyu (2020). Trends in the incidence of diabetes mellitus: results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health, 20:1415.
Mensh, B.D., N.A. Wisniewski, B.M. Neil, and D.R. Burnett (2013). Susceptibility of interstitial continuous glucose monitor performance to sleeping position. J. Diab. Sci. Technol. 7:863–870.
Miller, K.M., R.W. Beck, N.C. Foster, and D.M. Maahs (2020). HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socioeconomic status on HbA1c in the T1D exchange clinic registry findings. Diab. Technol. Therapeut. 22:645–650.
Moser, O., M. Dietrich, O. McCarthy, R.M. Bracken, and M.L. Eckstein (2020a). Bolus insulin dose depends on previous‐day race intensity during 5 days of professional road‐cycle racing in athletes with type 1 diabetes: A prospective observational study. Diab. Obes. Metab. 22:1714–1721.
Moser, O., A. Mueller, M.L. Eckstein, H. Ziko, F. Aberer, G. Treiber, C. Unteregger, H. Kojzar, J.K. Mader, C. Sourij, P. Pferschy, A. Obermayer, N. Tripolt, and H. Sourij (2020b). Improved glycaemic variability and basal insulin dose reduction during a running competition in recreationally active adults with type 1 diabetes—A single-centre, prospective, controlled observational study. PLoS ONE 15:e0239091.
Moser, O., M.C. Riddell, M.L. Eckstein, P. Adolfsson, R. Rabasa-Lhoret, L. Boom, P. van den, Gillard, K. Nørgaard, N.S. Oliver, D.P. Zaharieva, T. Battelino, C. Beaufort, R.M. de Bergenstal, B. Buckingham, E. Cengiz, A. Deeb, T. Heise, S. Heller, A.J. Kowalski, L. Leelarathna, C. Mathieu, C. Stettler, M. Tauschmann, H. Thabit, E.G. Wilmot, H. Sourij, C.E. Smart, P.G. Jacobs, R.M. Bracken, and J.K. Mader (2020c). Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 63:2501–2520.
Pitt, J.P., R.M. Bracken, S.N. Scott, F.Y. Fontana, K. Skroce, and O. McCarthy (2022). Nutritional intake when cycling under racing and training conditions in professional male cyclists with type 1 diabetes. J. Sports Sci. 40:1912–1918.
Prahalad, P., H. Hardison, O. Odugbesan, S. Lyons, M. Alwazeer, A. Neyman, B. Miyazaki, K. Cossen, S. Hsieh, D. Eng, A. Roberts, M.A. Clements, O. Ebekozien, and Collaborative, T. E. Q. I. (2024). Benchmarking diabetes technology use among 21 U.S. pediatric diabetes centers. Clin. Diab. 42:27–33.
Ratjen, I., K. Weber, M. Roden, M.-E. Herrmann, and K. Müssig (2015). Type 1 diabetes mellitus and exercise in competitive athletes. Exp. Clin. Endocrinol. Diab. 123:419– 422.
Rickels, M.R., S.N. DuBose, E. Toschi, R.W. Beck, A.S. Verdejo, H. Wolpert, M.J. Cummins, B. Newswanger, and M.C. Riddell; T1D Exchange Mini-Dose Glucagon Exercise Study Group (2018). Mini-dose glucagon as a novel approach to prevent exercise-induced hypoglycemia in type 1 diabetes. Diab. Care 41:1909–1916.
Riddell, M.C. and A.L. Peters (2023). Exercise in adults with type 1 diabetes mellitus. Nat. Rev. Endocrinol. 19:98–111.
Riddell, M.C., I.W. Gallen, C.E. Smart, C.E. Taplin, P. Adolfsson, A.N. Lumb, A. Kowalski, R. Rabasa-Lhoret, R.J. McCrimmon, C. Hume, F. Annan, P.A. Fournier, C. Graham, B. Bode, P. Galassetti, T.W. Jones, I.S. Millán, T. Heise, A.L. Peters, A. Petz, and L.M. Laffel (2017). Exercise management in type 1 diabetes: a consensus statement. Lancet Diab. Endocrinol. 5:377–390.
Riddell, M.C., D.P. Zaharieva, M. Tansey, E. Tsalikian, G. Admon, Z. Li, C. Kollman, and R.W. Beck (2019). Individual glucose responses to prolonged moderate intensity aerobic exercise in adolescents with type 1 diabetes: The higher they start, the harder they fall. Pediat. Diab. 20:99–106.
Riddell, M.C., S.N. Scott, P.A. Fournier, S.R. Colberg, I.W. Gallen, O. Moser, C. Stettler, J.E. Yardley, D.P. Zaharieva, P. Adolfsson, and R.M. Bracken (2020). The competitive athlete with type 1 diabetes. Diabetologia 63:1475–1490.
Rogers, M.A.M., C. Kim, T. Banerjee, and J.M. Lee (2017). Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 15:199.
Sacks, D.B., M. Arnold, G.L. Bakris, D.E. Bruns, A.R. Horvath, A. Lernmark, B.E. Metzger, D.M. Nathan, and M.S. Kirkman (2023). Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diab. Care 46:e151–e199.
Schmidt, S., M.B. Christensen, N. Serifovski, C. Damm‐Frydenberg, J.B. Jensen, T. Fløyel, J. Størling, A. Ranjan, and K. Nørgaard (2019). Low versus high carbohydrate diet in type 1 diabetes: A 12‐week randomized open‐label crossover study. Diab. Obes. Metab. 21:1680–1688.
Scott, S., P. Kempf, L. Bally, and C. Stettler (2019). Carbohydrate intake in the context of exercise in people with type 1 diabetes. Nutrients 11:3017.
Scott, S.N., M.P. Christiansen, F.Y. Fontana, C. Stettler, R.M. Bracken, C.A. Hayes, M. Fisher, B. Bode, P.H. Lagrou, P. Southerland, and M.C. Riddell (2020). Evaluation of factors related to glycemic management in professional cyclists with type 1 diabetes over a 7-day stage race. Diab. Care 43:1142–1145.
Scott, S.N., F.Y. Fontana, M. Cocks, J.P. Morton, A. Jeukendrup, R. Dragulin, J.F.P. Wojtaszewski, J. Jensen, R. Castol, M.C. Riddell, C. Stettler, and Diabetes, study of I. B. of E. (2021). Post-exercise recovery for the endurance athlete with type 1 diabetes: a consensus statement. Lancet Diab. Endocrinol. 9:304–317.
Skroce, K., A. Zignoli, F.Y. Fontana, F.M. Maturana, D. Lipman, A. Tryfonos, M.C. Riddell, and H.C. Zisser (2024). Real world interstitial glucose profiles of a large cohort of physically active men and women. Sensors 24:744.
Wadwa, R.P., L.M. Laffel, V.N. Shah, and S.K. Garg (2018). Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diab. Technol. Therapeut. 20: 395–402.
Weenen, E., N. van, Banholzer, S. Föll, T. Zueger, F.Y. Fontana, K. Skroce, C. Hayes, M. Kraus, S. Feuerriegel, V. Lehmann, S.N. Scott, F. Wortmann, and C. Stettler (2023). Glycaemic patterns of male professional athletes with type 1 diabetes during exercise, recovery and sleep: Retrospective, observational study over an entire competitive season. Diab. Obes. Metab. 25:2616–2625.
Zaharieva, D.P., K. Turksoy, S.M. McGaugh, R. Pooni, T. Vienneau, T. Ly, and M.C. Riddell (2019). Lag time remains with newer real-time continuous glucose monitoring technology during aerobic exercise in adults living with type 1 diabetes. Diab. Technol. Therapeut. 21:313–321.