KEY POINTS
- While total energy needs of female athletes are lower than male athletes, macronutrient needs tend to be similar relative to body size (i.e. g per kg body mass).
- Macronutrient utilization may vary over the course of a menstrual cycle; however, the overall effect appears to be small and can be minimized with proper fueling.
- Micronutrient needs of female athletes differ from male athletes, and the risk of deficiency should be considered in the development of nutrition plans.
- Nutrition programs that encourage autonomy, build competence and enhance connection have the potential to improve athlete performance by increasing intrinsic (internal) motivation.
- Nutrition plans should consider the individual needs of female athletes and be adjusted throughout the training and competitive phase to help athletes achieve their goals.
INTRODUCTION
Female athletes have physical and psychological attributes that differ from male athletes. While the various aspects of the menstrual cycle are perhaps the most often-considered distinction between cis-gendered female and male athletes, female athletes also exhibit physiological differences from males. These include, but not limited to, lower levels of testosterone (West et al., 2012), lower muscle mass (Janssen et al., 2000), differences in bone mineral density (Baker et al., 2020) and greater risk of reduced iron stores and lower hemoglobin concentrations (Peeling et al., 2008; Sandstrom et al., 2012). Female athletes tend to experience higher rates of disordered eating behaviors and eating disorders than male athletes (Sundgot-Borgen & Torstveit, 2004; Torstveit et al., 2008), and may be more susceptible to problematic consequences associated with low energy availability (LEA) (Tenforde et al., 2016).
Hence, a well-designed nutrition program for female athletes has the potential to:
- Reduce the risk of, and/or treat nutrient deficiencies, especially for nutrients of concern.
- Support training adaptations, with consideration to the distinct elements of the menstrual cycle and hormonal milieu experienced by females over the lifespan.
- Ensure adequate energy availability, and/or limit periods of problematic LEA, to support physical health and performance.
- Underpin successful performance in competition; and
- Enhance physical and emotional health and well-being.
A supportive nutrition program that is athlete-centered can encourage positive body image and a healthy relationship with food, while also enhancing athletic performance. When possible, programs should be customized to individual athlete needs, evaluated systematically and adjusted over time.
This Sports Science Exchange (SSE) article will review nutritional considerations that are specific to female athletes, while also outlining assessment and monitoring practices that may be of particular relevance to females. Special consideration will be given to behavioral approaches that can increase the success of nutrition plans or interventions, illustrated by a case study demonstrating the process of adapting a nutrition care plan over several competitive seasons.
NUTRIENT NEEDS OF FEMALE ATHLETES: CURRENT UNDERSTANDING
While a detailed analysis of female-male differences in physiology and response to exercise is beyond the scope of this discussion, the following serves as a brief overview of some of the nutrients of concern in the development of a nutrition plan:
Carbohydrate
Female athletes appear to be able to store similar amounts of glycogen as male athletes, so long as sufficient carbohydrate (8-12 g·kg-1·d-1) is consumed (Thomas et al., 2016). Studies examining the impact of menstrual cycle phase on glycogen resynthesis in the days following glycogen-depleting exercise have observed that glycogen storage is ~12-25% lower in the mid-follicular phase vs. the mid-luteal phase (Hackney, 1990; McLay et al., 2007; Nicklas et al., 1989). Notably, in these studies, carbohydrate intake ranged from 4 to 5.2 g·kg-1·day-1, which may be inadequate to support optimal glycogen storage. When a higher quantity of carbohydrate is consumed (8.4 g·kg-1·day-1), glycogen stores appear to be similar between phases (McLay et al., 2007). As such, it is suggested that female athletes who menstruate may benefit from prioritizing dietary carbohydrate during the follicular phase, especially when glycogen availability has the potential to affect performance of training sessions or competition (Moore et al., 2022).
Protein
Protein requirements for female athletes, based on studies examining the impact of dietary protein on myofibrillar protein synthesis (MPS) after exercise, suggest that, on a gram-per-kilogram body mass basis, protein requirements for female athletes are similar to male athletes (Thomas et al., 2016; West et al., 2012). The higher estrogen-to-progesterone ratio of the mid-follicular phase theoretically exerts a possible protein-sparing effect that may be of benefit to female athletes who menstruate and are not on oral contraceptives (Lamont et al., 2001; Lariviere et al., 1994). However, MPS did not differ between the follicular and luteal phases when 0.37 g·kg-1 of protein were provided after resistance training (Miller et al., 2006). This suggests that hormone-mediated differences in MPS are minimized when adequate dietary protein is available.
Hydration and Fluids
Both estrogen and progesterone can influence fluid balance. During the luteal phase, elevations in both hormones can increase interstitial fluid, potentially reducing plasma volume and increasing osmolality (Giersch et al., 2020). This suggests that there is greater potential for compromised hydration in the luteal phase, especially when exercise is prolonged or is undertaken in hot environments. To date, however, studies have not thoroughly examined the impact of menstrual cycle phase or oral contraceptive use on hydration status and/or athletic performance.
Iron
Iron deficiency is reported to affect ~35% of female athletes (Peeling et al., 2008). Foot strike hemolysis, gastrointestinal blood losses, altitude training and inflammatory responses associated with training are each associated with increased iron turnover (Pedlar et al., 2018; Peeling et al., 2008). Menstrual blood losses and withdrawal bleeds for those using oral contraceptives also increase iron requirements. Reduced iron stores may be associated with impairments in performance, even in the absence of frank anemia (DellaValle & Haas, 2011, 2014; Friedmann et al., 2001; Hinton et al., 2000). As such, iron supplementation and increased dietary intake of iron are common recommendations for female athletes (Peeling et al., 2023). While estrogen may exert an iron-sparing effect by downregulating the hormone hepcidin during the late follicular phase of the menstrual cycle, the impact of manipulating iron intake over the menstrual cycle has not been rigorously tested (Yang et al., 2012).
CONSIDERATIONS FOR THE DEVELOPMENT OF NUTRITION PLANS FOR FEMALE ATHLETES
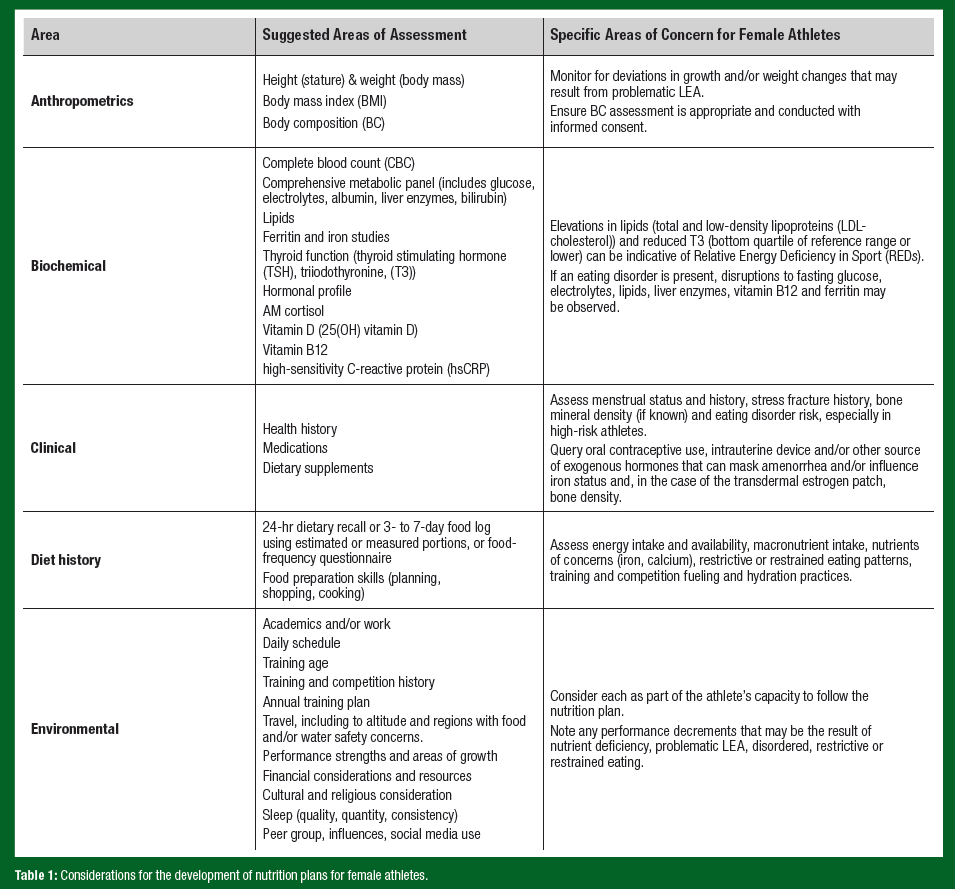
Effective, well-designed nutrition plans should take an athlete-centered approach. Table 1 outlines possible considerations when developing a nutrition plan for a female athlete, following a modified “ABCDE” approach (A – anthropometry, B – biochemistry, C – clinical, D – dietary intakes, E – environment and evaluation) (Sundgot-Borgen et al., 2013). Note that this list should be considered suggestive and not exhaustive.
EMERGING ISSUES AND CONSIDERATIONS
Oral Contraceptive and/or Exogenous Hormone Use
Athletes may, for a variety of reasons, use oral contraceptive or exogenous hormones (intrauterine device, hormonal patch), including to prevent pregnancy, manage menstrual symptoms, reduce menstrual blood losses, align bleeding days with competition or to avoid menstrual bleeds entirely. The use of the oral contraceptive pill might impact training adaptations, though these effects appear to be modest (Elliott- Sale et al., 2020). While the concept of adjusting training to menstrual cycle phase has gained popularity in recent years, current evidence does not support this practice. Indeed, in a recent systematic review and meta-analysis, McNulty et al. (2020) observed a trivial reduction of exercise performance in the follicular phase of the menstrual cycle, however, the quality of evidence was deemed “low”, and the between-study variation was large, suggesting that the individual athlete’s response across the menstrual cycle should drive coach and athlete decisions to adapt training.
Relative Energy Deficiency in Sport (REDs) Risk
In 2023, the International Olympic Committee issued updated guidelines on the assessment, monitoring and treatment of REDs (Mountjoy et al., 2023). The current model differs from previous iterations in the introduction of the concept of adaptive and problematic LEA. Whereas adaptive LEA tends to be short in duration (days to weeks) and does not lead to deleterious effects on health or performance, prolonged (weeks to months), severe and/or recurrent LEA can lead to the signs and symptoms of REDs (Mountjoy et al., 2023). The updated REDs Clinical Assessment Tool (REDs-CAT) outlines the risk for REDs, which is based on a four-color light system (green = none to very low risk for REDs to red = very high/extreme risk), and the diagnostic criteria. These include severe primary indicators such as primary amenorrhea or secondary amenorrhea lasting 12 months or more; primary indicators such as secondary hypothalamic amenorrhea of 3 to 11 months, one or more high-risk stress fractures or two or more low-risk bony stress injuries within the previous two years, reduced bone mineral density (Z-score < 1.0), bone loss or inadequate bone accrual, deviation from adolescent growth curve or elevated Eating Disorder Examination Questionnaire (EDE-Q) score; and secondary indicators such as oligomenorrhea, T3 hormone in the lowest quartile of the clinical reference range, depression or history of one low-risk BSI within the previous two years (Stellingwerff et al., 2023). Taken together, the clinical assessment allows for better diagnosis, treatment and monitoring of REDs.
Body Composition (BC) Assessment and Monitoring
Athletes may intentionally manipulate body weight with the aim of improving training outcomes or competitive success. Determining an “optimal” weight range for training or competition is nuanced, and may require a multi-season, interdisciplinary approach that considers:
- Stage of season, competitive cycle or career
- Performance metrics, derived from training and/or competition
- Menstrual function, including, if possible, menses and/or ovulation
- Blood work and other monitoring (e.g. bone mineral density)
- Eating behaviours
- Emotional health
- Athlete buy-in
Recently, Mathisen et al. (2023) published best practice recommendations for BC to reduce health and performance risks associated with the assessment and monitoring of BC. Among the recommendations are that athletes considered for BC assessment or manipulation are at the competitive national (Tier 3) level of their sport (McKay et al., 2022) and at least 18 years old. The process for BC assessment should be clearly outlined to the athlete, and informed consent should be obtained. Consent should be re-checked regularly, even if BC assessment is warranted for medical reasons. BC testing should not be undertaken if the athlete does not have reasonable access to a health and performance team, or there are concerns around eating behaviors or anxiety surrounding physique (Mathisen et al., 2023).
FUELING THE FEMALE ATHLETE: USING THE SOCIAL DETERMINATION FRAMEWORK
According to the Social Determination Theory (Ryan & Deci, 2020), individuals are more likely to generate consistent behavior change if they have autonomy, competence and connection. Autonomy refers to an athlete who feels in control of their behaviors and goals. To achieve competence, an athlete or individual should be supported as they gain mastery of tasks and learn different skills. Connection or relatedness provides a sense of belonging and attachment to others (Ryan & Deci, 2000). When an individual feels that they have the skills needed for success, they are more likely to take actions that will help them achieve their goals. Individuals with an internal sense of control have been found to be more likely to maintain a diet or exercise routine (Cobb-Clark et al., 2014). In competitive settings, a self of self-determination can motivate people to excel, possibly by increasing persistence and the sense that the athlete can succeed and grow (Pelletier et al., 2001). Athletes who feel that they can achieve their goals and overcome challenges are often driven to perform better.
HYPOTHETICAL CASE STUDY
Leah is a hammer thrower training for the Olympics. She joined the national team as a former team-sport athlete with exceptional speed and power, but relatively low body mass for her event. Her BMI is 27.2 kg/m2, whereas normative data suggests that top world and Olympic finishers tend to have a BMI of > 30 kg/m2 (note that BMI is used in this case as normative data on throwers tends to include height and weight rather than BC). Her first possible Olympic Games is three years away. What sort of nutrition interventions should be prioritized?
Sample Interventions – Year 1
- Collaborate with Leah to develop a meal plan to meet her carbohydrate (6-7 g kg-1 day-1) and protein (1.8 g kg-1 day-1) requirements to support training and recovery (Moore et al., 2022; Sygo et al., 2019).
- Encourage Leah to consume a modest energy surplus to support lean mass and body mass gains (Garthe et al., 2011); provide sample meal plans and work with Leah to generate ideas to add extra calories to her meals.
- Conduct baseline blood work as part of Leah’s routine medical assessment. Provide nutrient supplementation to treat deficiency and ensure optimal levels are achieved to support health and performance (vitamin D, iron) (Peeling et al., 2023).
- Conduct baseline BC testing with Leah’s informed consent, with the primary aim to assess lean muscle mass. Convey to Leah that this can be part of routine monitoring of training adaptations and to evaluate the effectiveness of her training program. Share results first with Leah, and with her consent, with her coach and performance team.
Follow-up and Re-assessment – Year 2
After a year of working together, Leah does not achieve the weight or lean mass gains targeted at the start of the previous season. Despite this, her performance improves steadily over the season, and she sets a personal best at the national championships. You learn that Leah was not bringing her meals or recovery foods consistently to training and struggles with meal planning at home. She feels overburdened as she is in the final year of her undergraduate degree. Her coach requests you speak with Leah about introducing creatine supplementation to support her training.
Sample Interventions – Year 2
- Collaborate with Leah to simplify meal planning. Streamline her post-training recovery and provide her with new recipe ideas and cooking tips to make meal prep easier.
- Reaffirm the value and importance of eating regularly, both to support training adaptations and lean mass gains.
- Explain the potential benefits of creatine supplementation to Leah, and, with her consent, develop a safe and appropriate supplement plan.
Follow-up – Year 3
After experiencing some initial weight gain, Leah’s weight drops again by the end of the season. She admits that she stopped taking creatine as she feels bloated when she takes it. Over the course of several conversations, it becomes apparent that Leah is unconvinced that weight gain is necessary for her performance, and that she feels uncomfortable with the extra body weight. While she has always embraced being a muscular athlete, increasing her weight and seeing her body change has been more emotionally challenging than she expected.
Sample Interventions – Year 3
- You and Leah agree to focus on sound nutrition practices, good recovery and thoughtful planning for travel and competition rather than weight as the primary outcome of your work together. You agree to discontinue creatine for now, while continuing with vitamin D and iron supplements to ensure she is meeting her needs and preventing deficiency.
- You collaborate with Leah’s performance team (including her coach and mental performance coach) to develop strategies to manage the emotional discomfort associated with weight changes. You collaborate with her strength & conditioning coach to monitor associations between her weight and/or lean mass changes and performance outcomes (strength, speed, power, competition performance) to inform future planning.
Follow-up – Year 4
Satisfied with the revised plan, Leah qualifies for her first Olympic Games. At the Games, she gains valuable experience, but does not advance past the preliminary round. For the next year, you work on maintaining consistent routines, expanding Leah’s cooking skills and recipe repertoire and strategies to manage jet lag and frequent travel. You encourage her to continue to work with her mental performance coach and collaborate with her performance team to identify next steps to improve her performance. Leah finishes the season by making her first-ever world championship final, setting a personal best in the process.
Follow-up – Year 5
Upon returning from the world championships, Leah notes that she was one of the smaller competitors in the finals and is now motivated to follow your nutrition recommendations and increase her weight and strength. She resumes taking creatine, recognizing the potential benefit to her training and performance. You introduce beta-alanine to her supplement protocol, based on data suggesting potential benefits to her work in the weight room (Varanoske et al., 2017). Leah accepts this suggestion and is compliant with the plan. That summer, Leah sets the national record for her event, and finishes the season ranked fifth in the world. She finishes the season with a BMI of 29.7 kg/m2, and her highest-ever amount of lean mass.
Follow-up – Year 6
Leah enters the pre-Olympic year feeling highly motivated and clear about her goals. She becomes increasingly consistent with her nutrition routines and is proactive in asking questions and seeking support. Over the season, her weight continues to increase gradually, and by the summer, she has set personal bests in the weight room, and on the field, reaching a BMI of 30.9 kg/m2. She says she feels comfortable in her body and has learned to embrace the higher body weight. At the world championships, Leah competes brilliantly, earning a bronze medal.
Hypothetical Case Study: Key Take-Aways
While Leah’s lack of adherence to the nutrition plan in the early part of her career might have been concerning, this scenario emphasizes the benefits from supporting athletes to make autonomous decisions about their physical and mental well-being. By educating Leah and empowering her to make her own decisions about her career, Leah develops autonomy and competence. Listening sincerely to Leah’s concerns establishes a trusting relationship between practitioner and athlete. Taken together, the self-determination Leah experiences increases her intrinsic motivation and drive for success.
PRACTICAL APPLICATIONS
- When developing nutrition plans and programs for female athletes, practitioners should consider:
- The unique nutrition needs of female athletes, including key nutrients of concern.
- The physiological differences between male and female athletes, including, but not limited to, those associated with menstrual cycle phase and life stage.
- The psychological and/or emotional needs of female athletes, including risk factors for restrictive and disordered eating, physique anxiety and problematic LEA.
- Strategies to increase intrinsic motivation in female athletes, as well as other social determinants associated with successful performance.
SUMMARY
Female athletes benefit from individualized programs that meet their nutrition needs and health and performance goals. The skilled practitioner will use a variety of assessment and monitoring tools and resources to provide females with the best-possible chance to succeed in their training and competitive environment, and to align nutrition plans with the athlete’s evolving needs. Rather than a one-size-fits all approach, programs that recognize the unique needs of female athletes have the potential to improve both psychological health and emotional wellbeing of the athlete, as well as physical performance in training and competition.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc.
REFERENCES
Baker, B.S., Z. Chen, R.D. Larson, M.G. Bemben, and D.A. Bemben (2020). Sex differences in bone density, geometry, and bone strength of competitive soccer players. J. Musculoskelet. Neuronal Interact. 20:62-76.
Cobb-Clark, D.A., S.C. Kassenboehmer, and S. Schurer (2014). Healthy habits: The connection between diet, exercise, and locus of control. J. Econom. Behav. Organiz. 98:1-28.
DellaValle, D.M., and J.D. Haas (2011). Impact of iron depletion without anemia on performance in trained endurance athletes at the beginning of a training season: A study of female collegiate rowers. Int. J. Sport Nutr. Exerc. Metab. 21:501-506.
DellaValle, D.M., and J.D. Haas (2014). Iron supplementation improves energetic efficiency in iron-depleted female rowers. Med. Sci. Sports Exerc. 46:1204-1215.
Elliott-Sale, K.J., K.L. McNulty, P. Ansdell, S. Goodall, K.M. Hicks, K. Thomas, P.A. Swinton, and E. Dolan (2020). The effects of oral contraceptives on exercise performance in women: A systematic review and meta-analysis. Sports Med. 50:1785-1812.
Friedmann, B., E. Weller, H. Mairbaurl, and P. Bartsch (2001). Effects of iron repletion on blood volume and performance capacity in young athletes. Med. Sci. Sports Exerc. 33:741-746.
Garthe, I., T. Raastad, and J. Sundgot-Borgen (2011). Long-term effect of nutritional counselling on desired gain in body mass and lean body mass in elite athletes. Appl. Physiol. Nutr. Metab. 36:547-554.
Giersch, G.E.W., N. Charkoudian, R.L. Stearns, and D.J. Casa (2020). Fluid balance and hydration considerations for women: Review and future directions. Sports Med. 50:253-261.
Hackney, A.C. (1990). Effects of the menstrual cycle on resting muscle glycogen content. Horm. Metab. Res. 22:647.
Hinton, P.S., C. Giordano, T. Brownlie, and J.D. Haas (2000). Iron supplementation improves endurance after training in iron-depleted, nonanemic women. J. Appl. Physiol. 88:1103-1111.
Janssen, I., S.B. Heymsfield, Z.M. Wang, and R. Ross (2000). Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J. Appl. Physiol. 89:81-88.
Lamont, L.S., A.J. McCullough, and S.C. Kalhan (2001). Gender differences in leucine, but not lysine, kinetics. J. Appl. Physiol. 91:357-362.
Lariviere, F., R. Moussalli, and D.R. Garrel (1994). Increased leucine flux and leucine oxidation during the luteal phase of the menstrual cycle in women. Am. J. Physiol. 267:E422-E428.
Mathisen, T.F., T. Ackland, L.M. Burke, N. Constantini, J. Haudum, L.S. Macnaughton, N.L. Meyer, M. Mountjoy, G. Slater, and J. Sundgot-Borgen (2023). Best practice recommendations for body composition considerations in sport to reduce health and performance risks: A critical review, original survey and expert opinion by a subgroup of the IOC consensus on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 57:1148-1158.
McKay, A.K.A., T. Stellingwerff, E.S. Smith, D.T. Martin, I. Mujika, V.L. Goosey-Tolfrey, J. Sheppard, and L.M. Burke (2022). Defining training and performance caliber: A participant classification framework. Int. J. Sports Physiol. Perform. 17:317-331.
McLay, R.T., C.D. Thomson, S.M. Williams, and N.J. Rehrer (2007). Carbohydrate loading and female endurance athletes: effect of menstrual-cycle phase. Int. J. Sport Nutr. Exerc. Metab. 17:189-205.
McNulty, K.L., K.J. Elliott-Sale, E. Dolan, P.A. Swinton, P. Ansdell, S. Goodall, K. Thomas, and K.M. Hicks (2020). The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Med. 50:1813- 1827.
Miller, B.F., M. Hansen, J.L. Olesen, A. Flyvbjerg, P. Schwarz, J.A. Babraj, K. Smith, M.J. Rennie, and M. Kjaer (2006). No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am. J. Physiol. 290:E163-E168.
Moore, D.R., J. Sygo, and J.P. Morton (2022). Fuelling the female athlete: Carbohydrate and protein recommendations. Eur. J. Sport Sci. 22:684-696.
Mountjoy, M., K.E. Ackerman, D.M. Bailey, L.M. Burke, N. Constantini, A.C. Hackney, I.A. Heikura, A. Melin, A.M. Pensgaard, T. Stellingwerff, J.K. Sundgot-Borgen, M.K. Torstveit, A.U. Jacobsen, E. Verhagen, R. Budgett, L. Engebretsen, and U. Erdener (2023). 2023 International Olympic Committee's (IOC) consensus statement on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. 57:1073-1097.
Nicklas, B.J., A.C. Hackney, and R.L. Sharp (1989). The menstrual cycle and exercise: performance, muscle glycogen, and substrate responses. Int. J. Sports Med. 10:264- 269.
Pedlar, C.R., C. Brugnara, G. Bruinvels, and R. Burden. (2018). Iron balance and iron supplementation for the female athlete: A practical approach. Eur. J. Sport Sci. 18:295- 305.
Peeling, P., B. Dawson, C. Goodman, G. Landers, and D. Trinder (2008). Athletic induced iron deficiency: New insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 103:381-391.
Peeling, P., M. Sim, and A.K.A. McKay (2023). Considerations for the consumption of vitamin and mineral supplements in athlete populations. Sports Med. 53(Suppl 1):15-24.
Pelletier, L.G., M.S. Fortier, R.J. Vallerand, and N.M. Briere (2001). Associations among perceived autonomy support, forms of self-regulation, and persistence: a prospective study. Motiv. Emotion 25:279-306.
Ryan, R.M., and E.L. Deci (2020). Intrinsic and extrinsic motivation from a self-determination theory perspective: Definitions, theory, practices, and future directions. Contemp. Educ. Psychol. 61: 101860.
Sandstrom, G., M. Borjesson, and S. Rodjer (2012). Iron deficiency in adolescent female athletes - is iron status affected by regular sporting activity? Clin. J. Sport Med. 22:495-500.
Stellingwerff, T., M. Mountjoy, W.T. McCluskey, K.E. Ackerman, E. Verhagen, and I.A. Heikura (2023). Review of the scientific rationale, development and validation of the International Olympic Committee Relative Energy Deficiency in Sport Clinical Assessment Tool: V.2 (IOC REDs CAT2)-by a subgroup of the IOC consensus on REDs. Br. J. Sports Med. 57:1109-1118.
Sundgot-Borgen, J., and M.K. Torstveit (2004). Prevalence of eating disorders in elite athletes is higher than in the general population. Clin. J. Sport Med. 14:25-32.
Sundgot-Borgen, J., N.L. Meyer, T.G. Lohman, T.R. Ackland, R.J. Maughan, A.D. Stewart, and W. Muller (2013). How to minimise the health risks to athletes who compete in weight-sensitive sports review and position statement on behalf of the Ad Hoc Research Working Group on Body Composition, Health and Performance, under the auspices of the IOC Medical Commission. Br. J. Sports Med. 47:1012-1022.
Sygo, J., A. Kendig Glass, S.C. Killer, and T. Stellingwerff (2019). Fueling for the field: Nutrition for jumps, throws, and combined events. Int. J. Sport Nutr. Exerc. Metab. 29:95-105.
Tenforde, A.S., M.T. Barrack, A. Nattiv, and M. Fredericson (2016). Parallels with the female athlete triad in male athletes. Sports Med. 46:171-182.
Thomas, D.T., K.A. Erdman, and L.M. Burke (2016). Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Acad. Nutr. Diet. 116:501-528.
Torstveit, M.K., J.H. Rosenvinge, and J. Sundgot-Borgen (2008). Prevalence of eating disorders and the predictive power of risk models in female elite athletes: A controlled study. Scand. J. Med. Sci. Sports 18:108-118.
Varanoske, A.N., J.R. Hoffman, D.D. Church, R. Wang, K.M. Baker, S.J. Dodd, N.A. Coker, L.P. Oliveira, V.L. Dawson, D.H. Fukuda, and J.R. Stout (2017). Influence of skeletal muscle carnosine content on fatigue during repeated resistance exercise in recreationally active women. Nutrients 9:988.
West, D.W., N.A. Burd, T.A. Churchward-Venne, D.M. Camera, C.J. Mitchell, S.K. Baker, J.A. Hawley, V.G. Coffey, and S.M. Phillips (2012). Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J. Appl. Physiol. 112:1805- 1813.
Yang, Q., J. Jian, S. Katz, S.B. Abramson, and X. Huang (2012). 17beta-Estradiol inhibits iron hormone hepcidin through an estrogen responsive element half-site. Endocrinology 153:3170-3178.