KEY POINTS
- Scientific evidence supporting a role for omega-3 polyunsaturated fatty acids (O3FA) in promoting muscle hypertrophy and strength gains in athletes is weak at present, at least if the athlete follows standard sports nutrition guidelines to support muscle building, e.g., a positive energy balance and sufficient dietary protein intake.
- O3FA ingestion may facilitate the remodeling of skeletal muscle protein when the athlete is unable to consume or tolerate ingestion of an optimal per serving dose of protein (~0.3 g/kg body mass) during post-exercise recovery.
- Early evidence supports a prehabilitative/rehabilitative role of O3FA in maintaining muscle mass during short-term injury-induced muscle disuse that results in a prolonged period of limb immobilization, e.g., leg brace or leg cast. Case studies in a real-life injury setting are required to advance knowledge regarding the protective role of O3FA on muscle mass and quality during injury recovery.
- Initial findings do not support the idea that increasing O3FA ingestion will promote high-quality weight loss during short-term periods of energy restriction. This notion applies to athletes with the goal to reduce fat mass and preserve muscle mass such as weight-category sports, esthetic sports or sports that demand a particularly high power-to-mass ratio.
- Recent studies provide promising evidence regarding a role for O3FA in accelerating recovery from intense “muscle damaging” exercise, although further work is required across a range of sport-specific contexts.
- Further research is warranted to better understand the optimum dose of O3FA and the ratio of eicosapentaenoic acid (EPA) to docosahexaenoic acid (DHA) to best support muscle recovery in athletes, and across a range of performance contexts, e.g., energy restriction, injury-induced muscle disuse, muscle repair and recovery.
INTRODUCTION
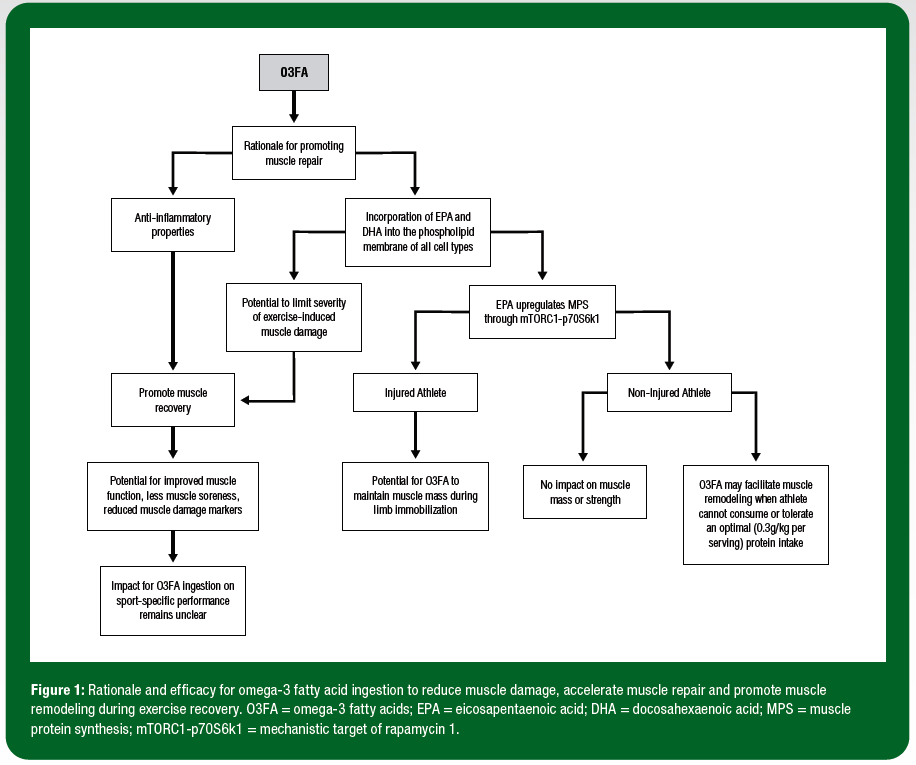
Omega-3 polyunsaturated fatty acids (O3FA) are a family of biologically active long-chain fatty acids. The most abundant and bioactive of the O3FA species are eicosapentaenoic acid (EPA; 20:5 n-3), docosahexaenoic acid (DHA; 22:6 n-3) and a-linolenic acid (ALA; 18:3 n-3) (Calder, 2015). Recently, O3FA have received considerable research attention in the context of athlete health and performance, particularly with regards to promoting muscle adaptation to exercise training and accelerating exercise recovery (Da Boit et al., 2017b; Philpott et al., 2019a). The scientific rationale that underpins the link between O3FA, muscle adaptation and exercise recovery stems from two distinct but likely interrelated known biological actions of O3FA (Calder, 2012). First, O3FA are readily incorporated into the phospholipid membrane of all cell types, including skeletal muscle cells, and serve to upregulate the activity of cell signaling pathways known to control the remodeling of muscle tissue (Gerling et al., 2019; McGlory et al., 2014). Second, the anti-inflammatory properties of O3FA serve as reasonable rationale to explore the efficacy of O3FA to accelerate the muscle repair process when the goal of the athlete is to promote recovery between training sessions (e.g., 2-3 training sessions/day) and/or during competitions (e.g., 4-8 hour intervals between heats, races or events or 2-4 day intervals between matches) (Calder, 2006). Given that these physiological processes underpin, (a) the muscle adaptive response to exercise training, and (b) the muscle repair process during exercise recovery, there is current interest in the applications of O3FA ingestion for sport performance (Figure 1).
Common food sources that are rich in O3FA include cold water fatty fish such as mackerel, sardines, salmon, trout and herring. The reader is referred to the Sports Science Exchange article by Rockwell & Ritz (2021) which provides a more in-depth overview of dietary food sources and supplementation with O3FA. The main purpose of this Sports Science Exchange article is to critically evaluate the scientific literature examining the efficacy of O3FA ingestion to promote athlete performance. We take a muscle-centric perspective, focusing on the role of O3FA in promoting skeletal muscle adaptation to exercise training and muscle repair during exercise recovery. This narrative is organized into three main sections. First, the role of O3FA in facilitating the muscle protein remodeling process that underpins muscle hypertrophy. Second, the role of O3FA in preserving lean body mass during catabolic conditions such as weight loss or injury-induced immobilization, and third, the role of O3FA in facilitating muscle repair during the acute exercise recovery period. The article concludes by highlighting existing gaps in knowledge and suggestions for future research directions related to O3FA, training adaptation and exercise recovery in athletes. As a note of caution, a limited number of studies in this field have been conducted in elite athlete populations. Therefore, critically evaluating the role of O3FA in promoting training adaptation and exercise recovery must primarily rely on extrapolating data from studies in recreationally trained or untrained humans, as well as animal studies and experiments conducted in cell culture (Da Boit et al., 2017a; McGlory et al., 2019b; Philpott et al., 2019a).
OMEGA-3 FATTY ACIDS and Muscle Hypertrophy
Muscle Protein Synthesis and Anabolic Cell Signaling
Skeletal muscle tissue is constantly being remodeled via the simultaneous metabolic processes of muscle protein synthesis (MPS) and muscle protein breakdown (MPB), collectively termed muscle protein turnover. This continual turnover of muscle proteins serves to degrade old and/or damaged muscle proteins, and synthesize new, more functional muscle proteins, to maintain skeletal muscle mass and quality. The primary metabolic driver of muscle hypertrophy, of particular relevance to strength/power-based athletes, is an increased stimulation of MPS, specifically of the contractile myofibrillar proteins (e.g., actin, myosin, tropomyosin), in response to resistance exercise training and protein feeding (McGlory & Phillips, 2014). The metabolic process of MPS is regulated at the molecular level by the activity of anabolic signaling proteins from the mechanistic target of rapamycin 1 (mTORC1) pathway that is housed inside the muscle cell itself (Kimball et al., 1998). Accordingly, nutritional strategies aimed at triggering this anabolic cell signaling cascade and maximizing the MPS response (and by extension the muscle hypertrophic response) to resistance exercise in athletic populations have primarily focused on manipulating the per meal/serving dose, source or timing of ingested protein (Witard et al., 2016). This body of work has provided, and continues to provide, considerable practical insight in terms of maximizing the muscle hypertrophic response to protein feeding.
A related, and perhaps more contemporary, topic of interest regarding the nutritional modulation of muscle mass concerns the interaction of protein with other nutrients in terms of increasing the utilization of ingested protein for MPS. While the capacity for carbohydrate to enhance the MPS response to ingested protein has been studied in detail (Staples et al., 2011), the role of fatty acids in increasing the utilization of ingested protein for MPS has only recently received attention. In this regard, findings from two seminal experimental studies in younger and older adults sparked considerable interest in the potential “muscle anabolic action” of O3FA (Smith et al., 2011a; Smith et al., 2011b). These proof-of-principle (i.e., set up to test a working hypothesis rather than mimic a real-world setting), tightly controlled, acute metabolic studies measured rates of MPS under basal (i.e., fasted and rested) and simulated fed conditions before and after 8 wk of fish oil (4 g/d) derived O3FA supplementation (1.86 g/d EPA, 1.50 g/d DHA). Amino acids and insulin were infused intravenously into the bloodstream to mimic the ingestion of a protein-rich, mixed macronutrient, meal. Whereas the basal response of MPS was not modulated by O3FA, the feeding-induced stimulation of MPS was enhanced by 30–60% following O3FA supplementation. Moreover, the phosphorylation status of the intramuscular cell signalling proteins known to up-regulate MPS (e.g., mTORC1-p70S6k1) was increased in response to simulated feeding following O3FA supplementation. Another interesting observation, albeit based on data generated in vitro using cell models, suggests that EPA, rather than DHA, is the active O3FA species in upregulating MPS in response to an anabolic (leucine) stimulus (Kamolrat and Gray, 2013). Taken together, these preliminary data suggest that the anabolic action of O3FA is mediated via an indirect mechanism (rather than exerting a direct anabolic effect), with O3FA, and specifically EPA, sensitizing skeletal muscle to potent anabolic stimuli such as amino acids and insulin.
The mechanism most commonly proposed to explain this indirect “priming action” of O3FA in stimulating MPS involves the direct incorporation of O3FA into the phospholipid membrane of skeletal muscle. Consistent with this idea, a recent study reported an ~100% increase in the O3FA composition (primarily of EPA) of skeletal muscle phospholipids following 4 wk of high dose (~5 g/day; ~3 g EPA and ~2 g DHA) O3FA supplementation (McGlory et al., 2014)"}" id="1467244493">(McGlory et al., 2014). In this study, these structural modifications to the muscle cell phospholipid membrane also coincided with an increased phosphorylation of mTORC1, a nutrient-sensitive cell signaling protein, and focal adhesion kinase, a mechanically sensitive protein known to regulate MPS. In contrast, no changes in tumour necrosis factor-α or C reactive protein concentrations as systemic markers of inflammation were observed over an 8-week period of O3FA supplementation (Smith et al., 2011)"}" id="-2110573700">(Smith et al., 2011b). Hence, based on existing knowledge, the primary mechanism that underpins the potential anabolic action of O3FA relates to modifying the lipid profile of the muscle phospholipid membrane that subsequently upregulates the activity of anabolic cell signalling proteins, as opposed to an anti-inflammatory response. Exactly how this change in muscle phospholipid membrane composition upregulates anabolic cell signalling remains to be fully elucidated and warrants investigation from scientists with expertise in lipidomics (large-scale study of pathways and networks of cellular lipids) methodology.
In recent years, we (McGlory et al., 2016) and others (Lalia et al., 2017) have extended these proof-of-principle studies to investigate the anabolic potential of O3FA using experimental designs that more closely mimic the nutrition and training practices of elite athletes. Rather than administering amino acids and insulin intravenously to simulate feeding, anabolic stimuli included either an orally ingested dose of intact protein, a standardized mixed macronutrient meal and/or a resistance exercise session(s). Informed by observations from cell-based experiments (Kamolrat and Gray, 2013), these studies administered a high dose (3-5 g/d) fish oil supplementation protocol that was rich in EPA content. We demonstrated that 8 wk of fish oil (5 g/d) derived O3FA (3.5 g/d EPA) supplementation failed to modulate the response of MPS to ingesting a 30 g bolus of whey protein (0.35 g/kg body mass) under both rested and post-exercise conditions in trained young men (McGlory et al., 2016). Thus, when ingesting a protein dose known to stimulate a maximal response of MPS, O3FA supplementation appears to confer no advantage in terms of increasing the muscle anabolic response. As such, it is conceivable that the 30 g dose of ingested whey protein in this study saturated the muscle protein synthetic machinery (Witard et al, 2014). Therefore, future work is warranted to investigate the influence of O3FA ingestion on the response of MPS to ingesting a suboptimal protein dose. In terms of applied practice, these data may reveal a context-specific role for O3FA in facilitating skeletal muscle protein remodelling if the athlete is unable to tolerate ingesting an optimal (~0.3 g/kg) dose of protein during exercise recovery.
Muscle Mass and Strength
Although measurements of MPS provide the “gold standard” acute marker of muscle growth, the anabolic action of O3FA also has been investigated using longitudinal study designs (Da Boit et al., 2017a; Smith et al., 2015). These longitudinal studies directly measure changes in muscle mass and strength in response to a period of O3FA supplementation, as summarized in a recent systematic review (Heileson and Funderburk, 2020). The majority of these studies have been conducted in older adult cohorts and, on balance provide promising results, particularly in older women (Da Boit et al., 2017a; Smith et al., 2015). For instance, Da Boit et al. (2017a) reported that an improvement in muscle strength (but not muscle mass) following 18 wk of resistance training was enhanced with O3FA supplementation in older women. However, no such benefit of O3FA ingestion was observed in older men. Consistent with this observation, O3FA supplementation (2 g/d fish oil) during 13 wk of resistance training resulted in greater strength gains compared with training alone (Rodacki et al., 2012). It follows that future studies are warranted to confirm this apparent sex difference in the muscle adaptive response to resistance training with O3FA ingestion, and second, determine the mechanism(s) that underpins this apparent sexual dimorphism in response to O3FA ingestion.
In comparison to older adults, relatively few studies have measured changes in muscle mass and/or strength with O3FA supplementation in younger athletic populations. The existing evidence is less promising with negligible (Couet et al., 1997), or at best minimal (e.g. 0.2 - 0.5 kg) (Noreen et al., 2010), increases in lean body mass reported over a 3-6 wk period of O3FA supplementation in trained men and women. Moreover, we reported no improvements in strength or power when competitive male and female soccer players supplemented their diet with O3FA over a typical 4 wk training period (Gravina et al., 2017). Taken together, the notion that O3FA offer an effective nutritional strategy to enhance muscle anabolism is primarily supported by data generated in older, more compromised, adult populations that may be considered more resistant to the anabolic stimuli of resistance exercise and protein ingestion. In comparison, the efficacy of O3FA ingestion to promote the muscle hypertrophic response to exercise training in elite athletes appears to be weak, at least if the athlete follows standard sports nutrition guidelines to support muscle building, a positive energy balance and sufficient dietary protein intake.
OMEGA-3 FATTY ACIDS and MUSCLE MASS PRESERVATION During Catabolic Conditions
Injury-Induced Muscle Disuse
In recent years, interest in O3FA from a muscle-centric perspective has expanded to explore the “protective” role of O3FA during catabolic conditions (McGlory et al., 2019b). In terms of practical application, these studies may translate to the injured athlete (i.e., leg fracture) who is forced into an extended period of limb immobilization and subsequent muscle disuse. The muscle atrophy associated with periods of muscle disuse is due, in part, to an impaired stimulation of MPS in response to ingested protein (Wall et al., 2013), a concept often coined “muscle anabolic resistance.” Interestingly, female athletes appear to be more susceptible to periods of muscle disuse since they are ~3 times more likely to sustain anterior cruciate ligament injuries compared with their male counterparts (Prodromos et al., 2007).
Accordingly, an elegant recent study investigated the influence of O3FA (3 g/day EPA, 2 g/day DHA) supplementation on changes in muscle mass and rates of MPS following 2 wk of leg casting in trained young women (McGlory et al., 2019a). Leg lean mass was reduced by 6% from pre to post in the control (sunflower oil) group, however there was no change in leg lean mass at any time point in the O3FA group. Moreover, following 2 wk of rehabilitation, muscle volume returned to baseline levels with O3FA supplementation, but remained below baseline in the control group. Accompanying the retention of muscle volume during simulated muscle disuse atrophy was a greater response of MPS both at immediate cessation of leg immobilization and following 2 wk of remobilization. Interestingly, in this study O3FA supplementation conferred no protective effect on the decline in muscle strength. As such, these data substantiate a prehabilitative/rehabilitative role for O3FA during short-term periods of injury-induced muscle disuse that are particularly commonplace in many team-based sports. As a note of caution, this study was conducted in the context of “uncomplicated” muscle disuse atrophy without the inherent complications that accompany injury such as excessive inflammation and a magnified stress (i.e., high cortisol) response. Therefore, future studies that more closely mimic real-life injuries are warranted to fully elucidate the role of O3FA in preserving muscle mass during injury and will likely include more reactive (i.e., as soon as an athlete gets injured) case-study type research designs.
High-Quality Weight Loss
Another practical application of O3FA ingestion in the context of “protecting” muscle mass during a catabolic scenario, that concerns athletes with the goal of “high-quality weight loss,” defined here as the loss of fat mass and preservation of muscle mass during kilocalorie restriction (Witard et al., 2019). For instance, athletes from weight-category sports, sports that demand a particularly high power-to-mass ratio or esthetic sports are likely to take a keen interest in any potential “protective” effects of O3FA during catabolic periods of negative energy balance. Our recent study examined the influence of O3FA supplementation during 2 wk of negative energy balance on changes in lean body mass (LBM) and fat mass in resistance-trained athletes (Philpott et al., 2019b). Athletes (n=20) underwent 2 wk of 40% kilocalorie restriction with half of the participants supplementing with an O3FA beverage twice daily, and the other participants supplementing with a carbohydrate placebo, while continuing their habitual training programme. Following 2 wk of supplementation, participants lost similar amounts of body mass, LBM and fat mass irrespective of dietary condition. To our knowledge, this is the only study to date that has investigated the influence of O3FA on changes in body composition during weight loss in athletes. While these initial data do not support a role for increasing O3FA ingestion during periods of calorie restriction, future studies are warranted to examine the impact of O3FA ingestion on muscle atrophy over longer periods of energy restriction in athletes.
OMEGA-3 FATTY ACIDS and MUSCLE REPAIR
The initial 96 h period post exercise is commonly defined as the acute exercise recovery period and is considered crucial in optimizing subsequent athletic performance and minimizing risk of soft tissue injury. The biological rationale for investigating the influence of O3FA ingestion on acute exercise recovery may be considered two-fold. First, O3FA have the potential to limit the severity of muscle fibre damage incurred following high-intensity exercise that consists of repeated eccentric-based muscle contractions. In theory, the direct incorporation of EPA and DHA into the phospholipid membrane following O3FA ingestion serves to increase the structural integrity of the muscle cell membrane, thereby limiting the severity of exercise-induced muscle damage. Second, O3FA have the potential to accelerate the muscle repair process by dampening the inflammatory response to muscle damaging exercise (Calder, 2006).
Laboratory Studies
A series of laboratory studies have examined the influence of O3FA on muscle function, perceived muscle soreness and indirect markers of muscle damage (e.g., blood creatine kinase (CK) concentrations) during acute (0-72 h) recovery from eccentric-based exercise. Overall, study findings may be considered mixed with some (Jouris et al., 2011; Tartibian et al., 2010), but not all (McKinley-Barnard et al., 2018), studies reporting better maintenance of muscle function, less muscle soreness, and reduced muscle damage during exercise recovery with O3FA supplementation. These discrepant findings may be attributed to several factors, including differences in experimental design, variation in dosage (2 - 4 g/d) and duration (7 d – 8 wk) of O3FA supplementation.
Applied Studies
While laboratory-based studies provide moderate evidence for the role of O3FA in accelerating exercise recovery, the direct application of these data to athletes should be considered with caution for several reasons. First, these studies are typically performed in untrained subjects with a view to eliciting a maximal level of muscle damage from unaccustomed exercise. Second, the muscle damage protocol (isokinetic dynamometry) does not simulate sporting movements such as rapid changes of direction and/or sudden decelerations that cause muscle damage. Third, the practical relevance of muscle function measurements (using isokinetic dynamometry, etc.) to sport performance is weak.
In an attempt to address these limitations, we recently conducted more applied studies in sub-elite soccer players (Philpott et al., 2018) and professional rugby players (Black et al., 2018). During the 72 h period following the muscle damaging exercise, soccer players in the O3FA supplementation group reported reduced levels of muscle soreness. The O3FA supplementation group also experienced a reduction in blood CK concentrations compared to the placebo (whey protein or carbohydrate) conditions. As such, these data imply that O3FA supplementation protected the muscle cell from the muscle damage protocol and theoretically soccer players experienced reduced muscle damage during exercise. However, the application of this physiological response of O3FA supplementation to sport-specific performance remains unclear. Whereas an improvement in countermovement jump performance with O3FA supplementation was observed in professional Rugby Union players during 5 wk of preseason training, no impact of O3FA ingestion was reported on soccer performance tests such as the yo-yo intermittent recovery test (soccer-specific field test of aerobic capacity), or the Loughborough soccer passing test. Further research is warranted to investigate the impact of O3FA ingestion in a sport-specific context (i.e., following a simulated soccer match) across a range of sports.
SUMMARY AND PRACTICAL APPLICATIONS
In summary, considerable research attention has recently focused on the efficacy of O3FA ingestion to accelerate post-exercise recovery and promote muscle adaptation to exercise training. The evidence supporting the role of O3FA in promoting muscle mass and strength adaptations for athletes appears to be weak. Furthermore, initial findings do not support a role for increasing O3FA ingestion during periods of kilocalorie restriction when the goal of the athlete is high-quality weight loss. Early evidence supports the role of O3FA on muscle recovery and injury prevention/rehabilitation in athletic populations, but further work is required in a real-world context in elite athletes, i.e., case studies on injured athletes. Future research is also warranted to better understand the optimum dose of O3FA and/or ratio of EPA to DHA for muscle adaptation and recovery across a range of sporting contexts.
REFERENCES
Black, K.E., O.C. Witard, D. Baker, P. Healey, V. Lewis, F. Tavares, S. Christensen, T. Pease, and B. Smith (2018). Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. Eur. J. Sport. Sci. 18:1357-1367.
Calder, P.C. (2006). Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 75:197-202.
Calder, P.C. (2012). Mechanisms of action of (n-3) fatty acids. J. Nutr. 142:592S-599S.
Calder, P.C. (2015). Functional roles of fatty acids and their effects on human health. J. Parenter. Enteral Nutr. 39:18S-32S.
Couet, C., J. Delarue, P. Ritz, J.M. Antoine, and F. Lamisse (1997). Effect of dietary fish oil on body fat mass and basal fat oxidation in healthy adults. Int. J. Obes. Relat. Metab. Disord. 21:637-643.
Da Boit, M., R. Sibson, S. Sivasubramaniam, J.R. Meakin, C.A. Greig, R.M. Aspden, F. Thies, S. Jeromson, D.L. Hamilton, J.R. Speakman, C. Hambly, A.A. Mangoni, T. Preston, and S.R. Gray (2017a). Sex differences in the effect of fish-oil supplementation on the adaptive response to resistance exercise training in older people: a randomized controlled trial. Am. J. Clin. Nutr. 105:151-158.
Da Boit, M., A.M. Hunter, and S.R. Gray (2017b). Fit with good fat? The role of n-3 polyunsaturated fatty acids on exercise performance. Metabolism 66:45-54.
Gerling, C.J., K. Mukai, A. Chabowski, G.J.F. Heigenhauser, G.P. Holloway, L.L. Spriet, and S. Jannas-Vela (2019). Incorporation of omega-3 fatty acids into human skeletal muscle sarcolemmal and mitochondrial membranes following 12 weeks of fish oil supplementation. Front. Physiol. 10:348.
Gravina, L., F.F. Brown, L. Alexander, J. Dick, G. Bell, O.C. Witard, and S.D.R. Galloway (2017). n-3 fatty acid supplementation during 4 weeks of training leads to improved anaerobic endurance capacity, but not maximal strength, speed, or power in soccer players. Int. J. Sport Nutr. Exerc. Metab. 27:305-313.
Heileson, J.L., and L.K. Funderburk (2020). The effect of fish oil supplementation on the promotion and preservation of lean body mass, strength, and recovery from physiological stress in young, healthy adults: a systematic review. Nutr. Rev. 78:1001-1014.
Jouris, K.B., J.L. McDaniel, and E.P. Weiss (2011). The effect of omega-3 fatty acid supplementation on the inflammatory response to eccentric strength exercise. J. Sports Sci. Med. 10:432-438.
Kamolrat, T., and S.R. Gray (2013). The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem. Biophys. Res. Commun. 432:593-598.
Kimball, S.R., R.L. Horetsky, L.S. and Jefferson (1998). Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am. J. Physiol. 274:C221-C228.
Lalia, A.Z., S. Dasari, M.M. Robinson, H. Abid, D.M. Morse, K.A. Klaus, and I.R. Lanza (2017). Influence of omega-3 fatty acids on skeletal muscle protein metabolism and mitochondrial bioenergetics in older adults. Aging 9:1096-1129.
McGlory, C., and S.M. Phillips (2014). Assessing the regulation of skeletal muscle plasticity in response to protein ingestion and resistance exercise: recent developments. Curr. Opin. Clin. Nutr. Metab. Care 17: 412-417.
McGlory, C., S.D. Galloway, D.L. Hamilton, C. McClintock, L. Breen, J.R. Dick, J.G. Bell, and K.D. Tipton (2014). Temporal changes in human skeletal muscle and blood lipid composition with fish oil supplementation. Prostaglandins Leukot. Essent. Fatty Acids 90:199-206.
McGlory, C., S.L. Wardle, L.S. Macnaughton, O.C. Witard, F. Scott, J. Dick, J.G. Bell, S.M. Phillips, S.D. Galloway, D.L. Hamilton, and K.D. Tipton (2016). Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signalling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 4:e12715.
McGlory, C., S.H.M. Gorissen, M. Kamal, R. Bahniwal, A.J. Hector, S.K. Baker, A. Chabowski, and S.M. Phillips (2019a). Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 33:4586-4597.
McGlory, C., P.C. Calder, and E.A. Nunes (2019b). The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 6:144.
McKinley-Barnard, S.K., T.L. Andre, J.J. Gann, P.S. Hwang, and D.S. Willoughby (2018). Effectiveness of fish oil supplementation in attenuating exercise-induced muscle damage in women during midfollicular and midluteal menstrual phases. J. Strength Cond. Res. 32:1601-1612.
Noreen, E.E., M.J. Sass, M.L. Crowe, V.A. Pabon, J. Brandauer, and L.K. Averill (2010). Effects of supplemental fish oil on resting metabolic rate, body composition, and salivary cortisol in healthy adults. J. Int. Soc. Sports Nutr. 7:31.
Philpott, J.D., C. Donnelly, I.H. Walshe, E.E. MacKinley, J. Dick, S.D.R. Galloway, K.D. Tipton, and O.C. Witard (2018). Adding fish oil to whey protein, leucine, and carbohydrate over a six-week supplementation period attenuates muscle soreness following eccentric exercise in competitive soccer players. Int. J. Sport Nutr. Exerc. Metab. 28:26-36.
Philpott, J.D., O.C. Witard, and S.D.R. Galloway (2019a). Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res. Sports Med. 27:219-237.
Philpott, J.D., N.J. Bootsma, N. Rodriguez-Sanchez, D.L. Hamilton, E. MacKinlay, J. Dick, S. Mettler, S.D.R. Galloway, K.D. Tipton, and O.C. Witard (2019b). Influence of fish oil-derived n-3 fatty acid supplementation on changes in body composition and muscle strength during short-term weight loss in resistance-trained men. Front. Nutr. 6:102.
Prodromos, C.C., Y. Han, J. Rogowski, B. Joyce, and K. Shi (2007). A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy 23:1320-1325.
Rodacki, C.L., A.L. Rodacki, G. Pereira, K. Naliwaiko, I. Coelho, D. Pequito, and L.C. Fernandes (2012). Fish-oil supplementation enhances the effects of strength training in elderly women. Am. J. Clin. Nutr. 95:428-436.
Smith, G.I., P. Atherton, D.N. Reeds, B.S. Mohammed, D. Rankin, M.J. Rennie, and B. Mittendorfer (2011a). Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am. J. Clin. Nutr. 93:402-412.
Smith, G.I., P. Atherton, D.N. Reeds, B.S. Mohammed, D. Rankin, M.J. Rennie, and B. Mittendorfer (2011b). Omega-3 polyunsaturated fatty acids augment the muscle protein anabolic response to hyperinsulinaemia-hyperaminoacidaemia in healthy young and middle-aged men and women. Clin. Sci. 121:267-278.
Smith, G.I., S. Julliand, D.N. Reeds, D.R. Sinacore, S. Klein, and B. Mittendorfer (2015). Fish oil-derived n-3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 102:115-122.
Staples, A.W., N.A. Burd, D.W. West, K.D. Currie, P.J. Atherton, D.R. Moore, M.J. Rennie, M.J. Macdonald, S.K. Baker, and S.M. Phillips (2011). Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Med. Sci. Sports Exerc. 43:1154-1161.
Tartibian, B., B.H. Maleki, and A. Abbasi (2010). The effects of omega-3 supplementation on pulmonary function of young wrestlers during intensive training. J. Sci. Med. Sport 13:281-286.
Wall, B.T., T. Snijders, J.M. Senden, C.L. Ottenbros, A.P. Gijsen, L.B. Verdijk, and L.J. van Loon (2013). Disuse impairs the muscle protein synthetic response to protein ingestion in healthy men. J. Clin. Endocrinol. Metab. 98:4872-4881.
Witard, O.C., S.R. Jackman, L. Breen, K. Smith, A. Selby, and K.D. Tipton, K.D. (2014). Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am. J. Clin. Nutr. 99:86-95.
Witard, O.C., S.L. Wardle, L.S. Macnaughton, A.B. Hodgson, and K.D. Tipton (2016). Protein considerations for optimising skeletal muscle mass in healthy young and older adults. Nutrients 8:181.
Witard, O.C., I. Garthe, and S.M. Phillips (2019). Dietary protein for training adaptation and body composition manipulation in track and field athletes. Int. J. Sport Nutr. Exerc. Metab. 29:165-174.