Stuart M. Phillips
KEY POINTS
- Muscle proteins turn over in response to both feeding and exercise and these responses are dictated by amino acid availability and exercise loading variables.
- Basal levels of muscle protein synthesis (MPS) are similar in young men and women as well as older men. However, older women appear to have an elevated rate of MPS.
- Younger men and women exhibit similar MPS responses to feeding, whereas older men and women show comparatively diminished MPS response, or become “resistant” to feeding, compared to their young counterparts. Older women in particular have a diminished MPS response to protein feeding.
- Younger women exhibit no difference in MPS across the menstrual cycle and very little difference in amino acid kinetics.
- Despite marked differences in the levels of testosterone, young men and women show very similar responses to resistance exercise.
- Older men have robust MPS responses to resistance exercise whereas older women are somewhat impaired in their ability to stimulate MPS with exercise.
- With resistance exercise training, MPS is elevated in both sexes and at all ages, but this does not change the feeding-induced increase in MPS.
- Sex-based differences in muscle protein turnover are not observed when comparing young men and women.
- With aging, changes in sex steroids in women (likely post-menopause) may result in an elevated rate of protein turnover, but with little response to feeding or contraction.
INTRODUCTION
It is well known that the ingestion of protein or amino acids results in increased plasma amino acid levels (hyperaminoacidemia) that stimulate muscle protein synthesis (MPS) and that resistance exercise increases this feeding-induced stimulation of MPS even more (Breen & Phillips, 2012). Various stimuli have been proposed to be responsible for the feeding-induced stimulation of MPS, including insulin mediated via changes in local blood flow (Fujita et al., 2006b, 2007), and leucine via stimulation of the mammalian target of rapamycin complex 1 (mTORC1) (Crozier et al., 2005). Many studies of the regulation of MPS in humans have been carried out in young men and a variety of nutritional and contractile variables have been studied. However, an understudied question is whether there are sex-based differences in MPS in response to feeding and muscle contractions. In addition, the role of aging in this area has been poorly studied with little data available. It is well documented that women have less lean muscle mass and more body fat than men (Mingrone et al., 2001). These differences in body composition are often thought to be due to differences in sex steroids and primarily testosterone. When testosterone is given exogenously in pharmacologic doses, it promotes the building of muscle or anabolism (Bhasin et al., 1996). Testosterone is thought to exert its anabolic effect through post-transcriptional mechanisms that stimulate MPS (Ferrando et al., 1998). There are, however, data suggesting that testosterone may inhibit muscle protein breakdown (Ferrando et al., 1998). In rodents, the administration of female sex steroids lowers MPS and thus may inhibit muscle growth (Toth et al., 2001). What is unclear, at least in humans, is whether male or female sex steroids within a normal physiological range exert an effect on muscle protein turnover. The purpose of this review is to summarize the state of our knowledge on sex-based differences in muscle protein turnover in response to both feeding and contraction.
BASAL MUSCLE PROTEIN TURNOVER
At rest in a basal fasted state, there appear to be few if any documented differences in MPS between younger men and women (Fujita et al., 2006a; Smith et al., 2009). For example, Fujita et al. (2006a) studied 10 men and eight women after an overnight fast and found no discernible differences in amino acid flux, intracellular turnover or proteolysis. The same authors (Fujita et al., 2006a) also observed no difference in basal muscle protein fractional synthesis rate, in accordance with other reports (Smith et al., 2009). Interestingly, and contrary to the supposition that testosterone may exert an anabolic effect, there may actually be a higher rate of MPS in women particularly as they age (Henderson et al., 2009; Smith et al., 2008, 2012b). Why the basal rate of MPS is higher in older women is unknown, but it may be that menopause is involved and that the chronic loss of estradiol and/or progesterone allows circulating androgens to exert their effects. These results are summarized in Figure 1.
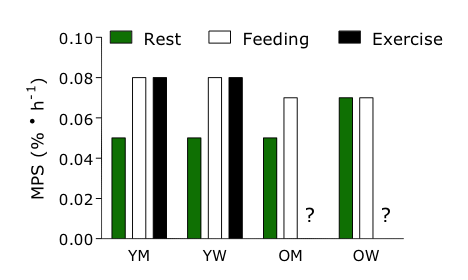
Figure 1. Relative changes in muscle protein synthesis (MPS) at rest (basal fasted) in young men (YM), young women (YW), older men (OM) and older women (OW). Responses of YM, YW, OM and OW to feeding (amino acids and/or protein) and resistance exercise (data not available for OM and OW) are also shown.
Another hormonally divergent “model” of human muscle protein turnover is that of the female menstrual cycle. Estrogen changes more than two-fold and progesterone changes by 10-20 fold across the cycle. The early follicular phase of the menstrual cycle is characterized by both low estrogen and progesterone, but during this phase estrogen increases, peaking at or near ovulation, whereas progesterone increases sharply in the luteal phase of the cycle. Nonetheless, despite marked variations in estrogen and progesterone, there are no differences in basal protein synthesis of either myofibrillar or connective tissue protein between the menstrual cycle phases (Miller et al., 2005). Interestingly, the use of oral contraceptives may lower basal MPS (Hansen et al., 2009a) and may also have a similar effect on collagen synthesis in tendon and muscle (Hansen et al., 2009b). However, it does not appear that long-term oral contraceptive use has a markedly negative influence on the adaptive responses to exercise in terms of performance (Rechichi et al., 2009; Vaiksaar et al., 2011).
RESPONSES TO FEEDING
Feeding of protein (or amino acids) induces a hyperaminoacidemia that normally stimulates MPS (Rennie et al., 2004) [Figure 1]. This stimulation appears to be due in part to an insulin-mediated increase in muscle blood flow (Fujita et al., 2006b, 2007) as well as the hyperaminoacidemia that independently stimulates MPS (Greenhaff et al., 2008). A key amino acid in stimulating MPS is leucine (Crozier et al., 2005). In comparisons of men and women, there appears to be little difference between how younger men and women respond to hyperaminoacidemia and hyperinsulinemia (Smith et al., 2009). In contrast, with aging, the elevated basal MPS described in elderly women (Henderson et al., 2009; Smith et al., 2008) actually results in a lower feeding-induced response of MPS compared to younger women (Smith et al., 2012a, b). While younger and older men have similar rates of MPS, the feeding-induced increase in MPS was found to be less in older men (Smith et al., 2012a). However, this is not something that is universally observed as a number of studies have shown equivalent and robust stimulation of MPS with protein feeding in young and older men (Kiskini et al., 2013; Symons et al., 2007). One possibility for the discrepant findings of protein-induced stimulation of MPS is that the selection of active vs. inactive elderly individuals may be having an impact. For example, periodic reductions in physical activity can reduce the MPS response to feeding and these responses are more common in older persons (Breen et al., 2013). Interestingly, even mild physical activity can restore the sensitivity of MPS to protein feeding in older adults (Fujita et al., 2007).
RESPONSES TO RESISTANCE EXERCISE
Resistance exercise is a robust stimulator of MPS (Breen & Phillips, 2012). The mechanism for stimulating MPS is still elusive but involves some form of mechanical events that link to and turn on the initiation of translation (making new protein) (Frey et al., 2009). The few comparisons of exercise-induced responses in MPS in men and women have reported no differences between the sexes. For example, West et al. (2012) compared the responses of MPS in young men and women after resistance exercise in the fed state and observed no differences either immediately or 24 h post-exercise. However, there were MPS response differences between the sexes in older adults who were engaging in a resistance training program, but it appeared to be a function of their baseline MPS rates (Smith et al., 2012b). In this study, MPS (both during basal postabsorptive conditions and during mixed meal intake) was measured before and after three months of exercise training in obese, 65-80 y old men and women. At the beginning of the study (before training) the basal, postabsorptive MPS rate was significantly greater in women than in men, whereas the meal-induced increase in MPS was greater in men than in women (Smith et al., 2012b). Exercise training resulted in a ~two-fold increase in their basal MPS but had no effect on the meal-induced increase in muscle protein fractional synthetic rate (P = 0.78). In women, exercise training increased MPS by ~40% and also had no effect on the meal-induced increase in MPS. These results are summarized in Figure 1.
It is possible that larger volumes of resistance exercise are required to initiate a robust stimulation of MPS in older persons vs. younger persons. Kumar et al. (2009) showed that there was a smaller response of MPS to exercises ranging in intensity from 60-90% of maximal in older men vs. young men. When older men completed a greater volume (twice as much) of resistance exercise, however, this lower MPS response in older men (Kumar et al., 2009) was overcome and found to be equivalent to that of young men.
RESPONSES TO RESISTANCE TRAINING
As some proof-of-principle that the responses of acute studies of MPS between men and women are valid, the results from training studies show very little if any difference between young men and women. To date, the largest study comparing men and women examined the hypertrophic response (increase in muscle fiber size) of the elbow flexors of men (n=243) and women (n=342) in response to 12 weeks of training (Hubal et al., 2005). They reported that the men experienced small but significantly (2.5%) greater gains in muscle cross-sectional area compared with women. Despite greater absolute gains in men, relative increases in strength measures were actually greater in women vs. men. Findings from other studies align with the general conclusion that if there are differences between men and women in resistance training-induced gains in muscle mass they are relatively small or non-existent (Abe et al., 2000), even with aging (Kosek et al., 2006; Leenders et al., 2013). Thus young women have the capacity to hypertrophy their muscle fibers in response to resistance training (Staron et al., 1989), despite a 10-20 fold lower testosterone concentration than men. This is consistent with the notion that local, rather than circulating systemic androgen hormone, mechanisms are dominant in promoting increases in MPS and fiber hypertrophy (West et al., 2010). An encouraging aspect of resistance training is that older men and women also retain the capacity to hypertrophy and gain strength and these responses appear to be of similar relative extent (Kosek et al., 2006; Leenders et al., 2013).
SEX-BASED DIFFERENCES IN PROTEIN METABOLISM
Sex-based differences in protein metabolism with dynamic exercise are relatively small when compared to differences in carbohydrate and lipid metabolism (Tarnopolsky, 2000, 2008; Tarnopolsky & Ruby, 2001). Nonetheless, some studies have been conducted in which men and women have been compared and have shown, in general, that women rely less on protein as a substrate during aerobic exercise than their matched male counterparts (Lamont et al., 2001, 2003; Phillips et al., 1993). A single study suggests that across the menstrual cycle there are relatively small changes in protein kinetics showing a potentially greater utilization of protein during the luteal phase with exercise (Lamont et al., 1987). However, the differences seen were based on estimates of sweat and urinary urea nitrogen excretion with no insight into kinetic measures. Moreover, the differences, while significant, were small, indicating that acute differences in estrogen and progesterone do not appear to exert a tremendous influence on the use of protein during exercise (Lamont et al., 1987). Remarkably, muscle protein turnover rates in the basal state, when protein kinetic rates are normalized to lean mass, are virtually identical between men and women (Fujita et al., 2006a; Miller et al., 2005).
CONCLUSION
Differences in muscle protein turnover and protein kinetics in the basal state, with feeding, and with resistance and endurance exercise, appear more similar than they are divergent in young men and women. With aging, and likely as a consequence of menopause, older women show an elevated rate of MPS compared to men and are refractory to the effects of feeding, to which older men show a relatively robust response. Despite large differences in sex steroid concentrations there is scant evidence to suggest that women, young or old, exhibit a markedly divergent response to resistance exercise training in terms of relative hypertrophy or strength gains. While there is evidence that women do rely slightly less on protein oxidation during aerobic work these differences are small and likely a function of lean body mass.
PRACTICAL APPLICATIONS
- Young men and women exhibit very little difference with respect to MPS in the basal state, in response to feeding or resistance exercise; thus, general recommendations to optimize lean mass gains in young men and women are similar.
- Young men have only slightly greater hypertrophic (increased muscle fiber size) responses to resistance training than young women and the relative gains in strength are similar or favor younger women.
- Older women have elevated basal rates of protein synthesis compared to older men and they do not exhibit as robust a response to feeding as do older men; the reasons for these differences remain unknown.
- While older men and women have never been directly compared as to MPS responses to resistance exercise, results from resistance training studies show relatively similar degrees of hypertrophy and strength gains.
- While young women appear to oxidize less protein during endurance exercise than do young men this difference is small and appears related to differences in lean body mass.
Figure Legend
Figure 1. Relative changes in muscle protein synthesis (MPS) at rest (basal fasted) in young men (YM), young women (YW), older men (OM) and older women (OW). Responses of YM, YW, OM and OW to feeding (amino acids and/or protein) and resistance exercise (data not available for OM and OW) are also shown.
REFERENCES
Abe, T., D.V. DeHoyos, M.L. Pollock, and L. Garzarella (2000). Time course for strength and muscle thickness changes following upper and lower body resistance training in men and women. Eur. J Appl. Physiol 81: 174-180.
Bhasin, S., T.W. Storer, N. Berman, C. Callegari, B. Clevenger, J. Phillips, T.J. Bunnell, R. Tricker, A. Shirazi, and R. Casaburi (1996). The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. N. Engl. J. Med. 335: 1-7.
Breen, L., and S.M. Phillips (2012). Nutrient interaction for optimal protein anabolism in resistance exercise. Curr. Opin. Clin. Nutr. Metab. Care 15: 226-232.
Breen, L., K.A. Stokes, T.A. Churchward-Venne, D.R. Moore, S.K. Baker, K. Smith, P.J. Atherton, and S.M. Phillips (2013). Two weeks of reduced activity decreases leg lean mass and induces 'anabolic resistance' of myofibrillar protein synthesis in healthy elderly. J Clin. Endocrinol. Metab. 98: 2604-2612.
Crozier, S.J., S.R. Kimball, S.W. Emmert, J.C. Anthony, and L.S. Jefferson (2005). Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J. Nutr. 135: 376-382.
Ferrando, A.A., K.D. Tipton, D. Doyle, S.M. Phillips, J. Cortiella, and R.R. Wolfe (1998). Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am. J. Physiol. 275: E864-871.
Frey, J.W., E.E. Farley, T.K. O'Neil, T.J. Burkholder, and T.A. Hornberger (2009). Evidence that mechanosensors with distinct biomechanical properties allow for specificity in mechanotransduction. Biophys. J. 97: 347-356.
Fujita, S., B.B. Rasmussen, J.A. Bell, J.G. Cadenas, and E. Volpi (2006a). Basal muscle intracellular amino acid kinetics in women and men. Am. J. Physiol.292: E77-83.
Fujita, S., B.B. Rasmussen, J.G. Cadenas, J.J. Grady, and E. Volpi (2006b). Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am. J. Physiol. 291: E745-754.
Fujita, S., B.B. Rasmussen, J.G. Cadenas, M.J. Drummond, E.L. Glynn, F.R. Sattler, and E. Volpi (2007). Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes 56: 1615-1622.
Greenhaff, P.L., L. Karagounis, N. Peirce, E.J. Simpson, M. Hazell, R. Layfield, H. Wackerhage, K. Smith, P. Atherton, A. Selby, and M.J. Rennie (2008). Disassociation between the effects of amino acids and insulin on signalling, ubiquitin-ligases and protein turnover in human muscle. Am. J. Physiol. 295: E595-604.
Hansen, M., H. Langberg, L. Holm, B.F. Miller, S.G. Petersen, S. Doessing, D. Skovgaard, T. Trappe, and M. Kjaer (2009a). Effect of administration of oral contraceptives on the synthesis and breakdown of myofibrillar proteins in young women. Scand. J. Med. Sci. Sports. 21: 62-72.
Hansen, M., B.F. Miller, L. Holm, S. Doessing, S.G. Petersen, D. Skovgaard, J. Frystyk, A. Flyvbjerg, S. Koskinen, J. Pingel, M. Kjaer, and H. Langberg (2009b). Effect of administration of oral contraceptives in vivo on collagen synthesis in tendon and muscle connective tissue in young women. J Appl. Physiol. 106: 1435-1443.
Henderson, G.C., K. Dhatariya, G.C. Ford, K.A. Klaus, R. Basu, R.A. Rizza, M.D. Jensen, S. Khosla, P. O'Brien, and K.S. Nair (2009). Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 23: 631-641.
Hubal, M.J., H. Gordish-Dressman, P.D. Thompson, T.B. Price, E.P. Hoffman, T.J. Angelopoulos, P.M. Gordon, N.M. Moyna, L.S. Pescatello, P.S. Visich, R.F. Zoeller, R.L. Seip, and P.M. Clarkson (2005). Variability in muscle size and strength gain after unilateral resistance training. Med. Sci. Sports Exerc. 37: 964-972.
Kiskini, A., H.M. Hamer, B.T. Wall, B.B. Groen, L.A. de, J.A. Bakker, J.M. Senden, L.B. Verdijk, and L.J. van Loon (2013). The muscle protein synthetic response to the combined ingestion of protein and carbohydrate is not impaired in healthy older men. Age 35: 2389-2398.
Kosek, D.J., J.S. Kim, J.K. Petrella, J.M. Cross, and M.M. Bamman (2006). Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl. Physiol. 101: 531-544.
Kumar, V., A. Selby, D. Rankin, R. Patel, P. Atherton, W. Hildebrandt, J. Williams, K. Smith, O. Seynnes, N. Hiscock, and M.J. Rennie (2009). Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 587: 211-217.
Lamont L.S., P. W. Lemon, and B. C. Bruot (1987). Menstrual cycle and exercise effects on protein catabolism. Med Sci. Sports Exerc. 19: 106-110.
Lamont, L.S., A.J. McCullough, and S.C. Kalhan (2001). Gender differences in leucine, but not lysine, kinetics. J. Appl. Physiol. 91: 357-362.
Lamont, L.S., A.J. McCullough, and S.C. Kalhan (2003). Gender differences in the regulation of amino acid metabolism. J. Appl. Physiol. 95: 1259-1265.
Leenders, M., L.B. Verdijk, L. van der Hoeven, K.J. van, R. Nilwik, W.K. Wodzig, J.M. Senden, H.A. Keizer, and L.J. van Loon (2013). Protein supplementation during resistance-type exercise training in the elderly. Med. Sci. Sports Exerc. 45: 542-552.
Miller, B.F., M. Hansen, J.L. Olesen, A. Flyvbjerg, P. Schwarz, J.A. Babraj, K. Smith, M.J. Rennie, and M. Kjaer (2005). No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Am. J. Physiol. 290: E163-168.
Mingrone, G., S. Marino, A. DeGaetano, E. Capristo, S.B. Heymsfield, G. Gasbarrini, and A.V. Greco (2001). Different limit to the body's ability of increasing fat-free mass. Metabolism 50: 1004-1007.
Phillips, S.M., S.A. Atkinson, M.A. Tarnopolsky, and J.D. MacDougall (1993). Gender differences in leucine kinetics and nitrogen balance in endurance athletes. J. Appl. Physiol. 75: 2134-2141.
Rechichi, C., B. Dawson, and C. Goodman (2009). Athletic performance and the oral contraceptive. Int. J. Sports Physiol. Perform. 4: 151-162.
Rennie, M.J., H. Wackerhage, E.E. Spangenburg, and F.W. Booth (2004). Control of the size of the human muscle mass. Annu. Rev. Physiol. 66: 799-828.
Smith, G.I., P. Atherton, D.T. Villareal, T.N. Frimel, D. Rankin, M.J. Rennie, and B. Mittendorfer (2008). Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65-80 year old men and women. PLoS. One.3, e1875.
Smith, G.I., P.J. Atherton, D.N. Reeds, B.S. Mohammed, H. Jaffrey, D. Rankin, M.J. Rennie, and B. Mittendorfer (2009). No major sex differences in muscle protein synthesis rates in the postabsorptive state and during hyperinsulinemia-hyperaminoacidemia in middle-aged adults. J. Appl. Physiol. 107: 1308-1315.
Smith, G.I., D.N. Reeds, A.M. Hall, K.T. Chambers, B.N. Finck, and B. Mittendorfer (2012a). Sexually dimorphic effect of aging on skeletal muscle protein synthesis. Biol. Sex Differ. 3: 11.
Smith, G.I., D.T. Villareal, D.R. Sinacore, K. Shah, and B. Mittendorfer (2012b). Muscle protein synthesis response to exercise training In obese, older men and women. Med. Sci. Sports Exerc. 44:1259-1266.
Staron, R.S., E.S. Malicky, M.J. Leonardi, J.E. Falkel, F.C. Hagerman, and G.A. Dudley (1989). Muscle hypertrophy and fast fiber type conversions in heavy resistance-trained women. Eur. J. Appl. Physiol. 60: 71-79.
Symons, T.B., S.E. Schutzler, T.L. Cocke, D.L. Chinkes, R.R. Wolfe, and D. Paddon-Jones (2007). Aging does not impair the anabolic response to a protein-rich meal. Am. J. Clin. Nutr. 86: 451-456.
Tarnopolsky, M.A. (2000). Gender differences in substrate metabolism during endurance exercise. Can. J. Appl. Physiol, 25: 312-327.
Tarnopolsky, M.A. (2008). Sex differences in exercise metabolism and the role of 17-beta estradiol. Med. Sci. Sports Exerc. 40: 648-654.
Tarnopolsky, M.A., and B.C. Ruby (2001). Sex differences in carbohydrate metabolism. Curr. Opin. Clin. Nutr. Metab. Care 4: 521-526.
Toth, M.J., E.T. Poehlman, D.E. Matthews, A. Tchernof, and M.J. MacCoss (2001). Effects of estradiol and progesterone on body composition, protein synthesis, and lipoprotein lipase in rats. Am. J. Physiol. 280: E496-501.
Vaiksaar, S., J. Jurimae, J. Maestu, P. Purge, S. Kalytka, L. Shakhlina, and T. Jurimae (2011). No effect of menstrual cycle phase and oral contraceptive use on endurance performance in rowers. J. Strength Cond. Res. 25: 1571-1578.
West, D.W., N.A. Burd, A.W. Staples, and S.M. Phillips (2010). Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int. J. Biochem. Cell Biol. 42: 1371-1375.
West, D.W., N.A. Burd, T.A. Churchward-Venne, D.M. Camera, C.J. Mitchell, S.K. Baker, J.A. Hawley, V.G. Coffey, and S.M. Phillips (2012). Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. J. Appl. Physiol. 112: 1805-1813.